Introduction
Obesity rates are increasing in developed and transition countries, becoming a worldwide epidemic [1]. In 2025, if the prevalence growth continues with this trend, the global figures of obesity will reach 18% in men and 21% in women [2]. In addition, it is well known that obesity is commonly accompanied by different comorbidities such as type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD), among others [3]. The prevalence of NAFLD is rising in parallel with the obesity epidemic, being worldwide one of the most important chronic liver diseases [4]. Currently, NAFLD is considered as a frequent component of the metabolic syndrome, which is closely related to inflammatory processes [5, 6]. The mechanisms underlying the progression of this disease are still largely unknown [7]. In any case, a number of studies have identified diverse NAFLD multifactorial determinants and causal interactions with genetic factors, oxidative stress, hormones secreted from the adipose tissue and systemic inflammation as well as unbalanced nutrition and unhealthy lifestyles [8]. Nowadays, the investigations are focusing on a novel liver biomarker, which has led to an important evolution over the past 8–10 years regarding suitable non-invasive diagnostic tools for fatty liver. However, most scores and indexes are not still fully valid in clinical practice [9]. Some studies have suggested M30 fragment (CK-18) and FGF-21 as potential biomarkers for NAFLD [10]. M30 fragment estimates hepatocyte apoptosis in NAFLD or non-alcoholic steatohepatitis (NASH), which is related to inflammation mechanisms and fibrosis stages [11, 12]. Another study [13] even suggested that CK-18 fragments (formed by M30 and M65 fragments) might be a useful biomarker to discriminate between NAFLD and NASH conditions. For instance, a recent review highlighted CK-18 as one of the more promising biomarkers to diagnosis NAFLD [14]. On the other hand, fibroblast growth factor 21 (FGF-21) is released from liver cells, from adipocytes and in other tissues [15]. A functional role for FGF-21 is to maintain the glucose and lipid homeostasis [16]. Previous studies have reported that in over-nutrition as well as in inflammatory processes, FGF-21 levels are increased [17]. Anti-inflammatory effects have been demonstrated to have beneficial results in obesity and comorbidities [18, 19]. In addition, levels of FGF-21 are elevated in NAFLD subjects and some investigators have found that FGF-21 is an independent biomarker to predict NAFLD [20]. Nowadays, the “gold standard” test to diagnosis NAFLD is the liver biopsy, for steatosis, fibrosis or cirrhosis [21]. Nevertheless, it is an invasive and unreliable procedure for many patients and therefore it is not frequently performed [22]. In this context, the treatment of NAFLD is based on general dietary weight loss strategies, with a lack of information about specific dietary approaches through well-designed randomized trials [23]. In this context, it is necessary to find suitable non-invasive biomarkers which, alone or in combination with other scores, are able to provide information to monitor metabolic disease such as NAFLD management in a personalized way [24, 25]. The present study aimed to evaluate the response of FGF-21 circulating levels to a weight loss intervention and their relationships with metabolic and inflammatory liver biomarkers such as the recently proposed liver disease biomarkers M30 fragment (CK-18 fragments) and plasminogen activator inhibitor-1 (PAI-I), in obese patients with metabolic syndrome.
Material and methods
Study design
The current study enrolled 66 participants of the RESMENA-S study, which was designed as a randomized, controlled intervention trial to compare the effects of two hypocaloric dietary strategies (American Heart Association and RESMENA) on metabolic syndrome features after a 6-month follow-up. Subjects were assigned (using the “random between 1 and 2” function in the Microsoft Office Excel 2003 software (Microsoft Iberica, Spain) to follow one of the two energy-restricted diets described elsewhere [26]. Both diets have been found to produce similar weight loss and metabolic outcomes, so both dietary groups were merged for the current analyses as statistically designed in other investigations of the RESMENA study [27]. Physical activity was evaluated by a 24 h questionnaire at the beginning and at the end of the study [26].
The RESMENA study followed the CONSORT 2010 guidelines, except for blinding. This research was performed according to the ethical guidelines of the Declaration of Helsinki, and it was registered. The trial was approved by the Research Ethics Committee of the University of Navarra (ref. 065/2009). Additional aspects of this intervention trial have been detailed elsewhere [28].
Nutritional intervention
Two hypocaloric dietary strategies, both with the same energy restriction (–30% of the individual’s requirements), were prescribed and evaluated. The reference diet was based on American Heart Association (AHA) guidelines, including 3–5 meals/day, a macronutrient distribution of 55% of total caloric value (TCV) from carbohydrates, 15% from proteins and 30% from lipids, a healthy fatty acid (FA) profile, a cholesterol consumption lower than 300 mg/day and a fiber intake of 20–25 g/day. Otherwise, the RESMENA diet was designed with a higher meal frequency, consisting of 7 meals/day, and a macronutrient distribution of 40% TCV from carbohydrates, 30% from proteins and 30% from lipids. The RESMENA diet also maintained a healthy fatty acid profile rich in omega-3, extra virgin olive oil intake, a cholesterol content of less than 300 mg/day and a fiber intake of 20–25 g/day. A 48-h weighed food record was completed at the beginning and at the end of the study, which was used to evaluate the volunteer’s adherence to the prescribed diet. The energy and nutrient content of these questionnaires were determined with the DIAL software (Alce Ingenieria, Madrid, Spain), as described elsewhere [26]. Anthropometric determinations were measured in fasting conditions and following standardized procedures previously reported [28]. Body composition was assessed by dual-energy X-ray absorptiometry (Lunar Prodigy, software version 6.0, Madison, WI), at baseline and at the end of the trial, according to validated protocols [26]. Body mass index (BMI) was calculated as the body weight divided by the squared height (kg/m2). Glucose, total cholesterol (TC), triglycerides (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (GGT) were measured on an autoanalyzer (Pentra C-200; HORIBA ABX, Madrid, Spain) with specific kits from this company. Plasma concentrations of CK18 fragment levels were assessed by M30® Apoptosense ELISA assay, cat. no. 10011 (PEVIVA, Nacka, Sweden) with an autoanalyzer system (Triturus, Grifols SA, Barcelona, Spain) following the manufacturer’s instructions. FGF-21 concentrations were analyzed using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Biovendor, Brno, Czech Republic) according to the supplier guidelines [29]. Fasting plasma levels of leptin and adiponectin were measured by ELISA using commercially available kits (Millipore, MA, USA). Plasma concentrations of high-sensitivity C-reactive protein (hsCRP) (Demeditec) were measured, and interleukin-6 (IL-6) (R&D Systems), tumor necrosis factor-α (TNF-α) (R&D Systems), and PAI-I (BioVendor) were measured with specific enzyme-linked immunosorbent assay kits from the specified suppliers using an autoanalyzer system (Triturus, Grifols SA, Barcelona, Spain) in accordance with the manufacturer’s instructions. Homeostatic model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of β cell function (HOMA-β) were estimated using the equation of Matthews et al. [30]. The fatty liver index (FLI) [31] is an algorithm derived from serum TG, BMI, waist circumference (WC) and GGT levels [32–35], which was validated in a large group of subjects with or without suspected liver disease with an accuracy of 0.84 (95% CI) in detecting a fatty liver condition. FLI varies between 0 and 100, the presence of steatosis being estimated when FLI score ≥ 60.
Statistical analysis
Analyses were performed using STATA version 12.0 (Stata Corp). According to previous studies and in the current investigations both diets (AHA and RESMENA) were equally beneficial and effective concerning weight loss and metabolic outcomes. For these the data were analyzed as one group. Changes in given variables were calculated as values of the end of the intervention less values at baseline. To analyze the changes of inflammation biomarkers during the intervention, the median value of the changes was used as a cut-off (above and below the 50th percentile) as previously applied [36]. Normality distributions of the evaluated variables were determined by means of the Shapiro-Wilk test. Continuous variables were compared between groups by Student’s t-test or the Mann-Whitney U test for parametric or non-parametric variables, respectively. The relationships among variables were assessed by Pearson’s correlation coefficient or Spearman’s rho (p), as appropriate depending on normality distributions. Multivariate linear regression models were fitted to assess the potential influence of liver-inflammation factors on FGF-21 levels after treatment and adjusted for age and gender. Two regression models were performed. Model 1 included changes after treatment in M30 and PAI-I. Model 2 also included changes in body weight. All p-values presented are two-tailed, and differences were considered statistically significant at p < 0.05.
Results
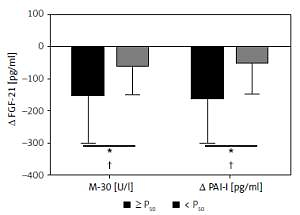
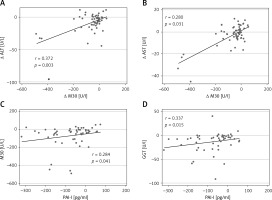
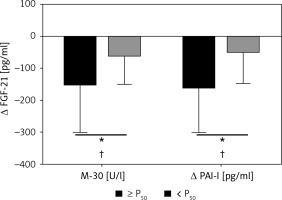
A total of 109 Caucasian adults were enrolled to participate in this study. Then, 93 participants started the dietary interventions after inclusion screening. They showed no statistically significant differences concerning anthropometric and biochemical measurements at the end of the 6 months. During follow-up there were patients who withdrew due to lack of adherence of the informed consent. The present study evaluated a subsample of 66 subjects (50.8 ±8.6; 36 M/30 F) who completed all the required data. Changes in anthropometric parameters and body composition as well as lipid and glucose metabolism values were assessed by standard procedures at baseline and after weight loss (Table I) in order to screen the general outcomes of the intervention. All measurements significantly improved at the end of the intervention. In addition, in order to characterize the evolution of hepatic markers and related liver inflammatory biomarkers paired t-tests were performed at baseline and after the nutritional follow-up. All hepatic and inflammatory markers also showed a significant clinical amelioration (Table II). In that sense, several linear regression analyses of data changes in FGF-21 and other inflammatory liver markers after the intervention period were fitted with the purpose of discriminating the relation with M30 (cell apoptosis marker) and PAI-I (plasminogen activator inhibitor). Some variables were independently studied by univariable linear regression. Therefore, variables potentially associated with FGF-21 were: Δ M30 (β = 0.26, R = 0.048, p = 0.066), Δ PAI-I (β = 0.665, R = 0.239, p < 0.001), Δ Age (β = –25.2, R = –0.008, p = 0.458). When these variables were jointly considered, the predictors of the model explained up to 24.1% (Table III) of the variation of the Δ FGF-21 in model 1 (adjusted R2 = 0.241, P model = 0.008). Likewise, the energy restricted nutritional intervention and subsequent weight lowering could affect this biomarker. Thus, in model 2 (Table III) the linear regression with the same outcomes was adjusted by Δ of weight loss, evidencing that the effect of variations of the inflammatory markers on FGF-21 is independent of the weight loss processes. In this context, the relationships between hepatic markers and inflammatory parameters were studied with the objective of seeking relevant clinical information. Thus, changes in FGF-21, M30 fragment and PAI-I as non-invasive liver markers with inflammatory biomarkers and metabolic status are also reported (Figures 1 and 2). Positive and significant associations were found between FGF-21 and M30 (r = 0.346; p = 0.012), GGT (r = 0.409; p = 0.003) and PAI (r = 0.521; p < 0.001). In addition, M30 showed significant relations with ALT (r = 0.372; p = 0.003) and AST (r = 0.280; p = 0.031). On the other hand, PAI-I showed significant associations with GGT (r = 0.337; p = 0.015) and M30 (r = 0.284; p = 0.041). Finally, in order to reinforce these results, Figure 3 illustrates that in accordance with P50 of Δ M30 and Δ PAI-I, the largest reductions in both inflammatory markers were linked with the largest decreases in Δ FGF-21.
Table I
Anthropometric and body composition, lipid and glucose metabolism of the participants after 6 months of dietary intervention to lose weight
Table II
Hepatic and inflammatory markers of the participants after 6 months of dietary intervention to lose weight
[i] Mean ± SD. Paired t-test was carried out. P < 0.05 was considered statistically significant. ALT – alanine transaminase, AST – aspartate transaminase, GGT – γ-glutamyl transferase, FLI – fatty liver index, M30 – M30 fragment of CK18, hs-CRP – high-sensitivity C-reactive protein, IL-6 – interleukin 6, PAI-1 – plasminogen activator inhibitor-1, FGF-21 – fibroblast growth factor 21.
Table III
Linear regression analyses according to changes in fibroblast growth factor 21 (FGF-21) involving different inflammatory liver markers after the 6-month intervention
Figure 1
Correlations of changes of fibroblast growth factor 21 (FGF-21) with changes of M30 (CK-18 fragment (A), plasminogen activator inhibitor (PAI-I) (B) and γ-glutamyl transferase (GGT) (C)

Figure 3
Changes in fibroblast growth factor 21 (FGF-21) according to the magnitude of changes in M30 and plasminogen activator inhibitor (PAI-I) categorized for median (≥ p50 or < p50). *P < 0.05 was considered statistically significant. +P < 0.05 was considered statistically significant after adjustment for changes in body weight
Discussion
NAFLD is becoming one of the most common liver diseases worldwide. Its complex pathogenesis is closely associated with metabolic disorders such as obesity [3, 37, 38]. To understand the specific mechanisms involved in this process is an urgent necessity [39]. Nowadays, molecules such as FGF-21 have emerged as potential players or biomarkers of the NAFLD pathogenesis in a framework of inflammation processes [40]. In this context, this study showed that the improvement in hepatic-inflammatory biomarkers induced by an energy restriction treatment prescribed to lose weight is associated with a concomitant reduction in FGF-21 circulating levels. Relevantly, this association was not directly related to weight loss, suggesting that the improvement in the inflammation induced by the dietary treatment is the most likely mechanism involved in the variation of FGF-21. Therefore, the current results highlight the relevance of FGF-21 as a non-invasive marker for prediction and management of liver disease. Several lines of evidence suggest that increased stress oxidation changes in several molecular factors, including adipokines, chemokines, and pro- or anti-inflammatory cytokines, are mainly involved in the progression of NAFLD/NASH [41]. Indeed, inflammation appears as a common feature with different stages of diverse liver disease, where specific assessment may help to better understand the progression of liver disturbances with specific inflammatory biomarkers such as FGF-21 or M30-frgaments. Regarding NAFLD treatment, dietary and lifestyle modifications are considered the first-step therapy for patients with NAFLD, but there is no consensus for pharmacological treatment [42]. It is important to take into account that this pathology has different grades of severity, which makes it necessary to develop appropriate biomarkers for its different stages [43]. For these reasons, current research has focused on identifying biomarkers to predict NASH or NAFLD [14, 44]. In this context, Bedogni et al. designed a simple scoring system named FLI, which includes TG, GGT, BMI, and WC, and is easily calculated. FLI was developed for the prediction of fatty liver disease (AUC = 0.84) [32]. The accuracy of FLI in comparison with the ultrasonography method for detection and quantification of hepatic steatosis has been validated in several countries [32]. Furthermore, previous studies have demonstrated and validated other non-invasive markers of liver status that were used in this investigation (M30 fragments) [45]. In addition, changes in recognized biochemical parameters, such us transaminases levels, are the most common non-invasive methodology to assess fatty liver. However, in patients with NAFLD more than 70% have normal liver enzyme values [46]. Early diagnosis of NAFLD is very important, but there is no single biochemical marker for the confirmation of NAFLD [47]. In this study, we evaluated the relationships between FGF-21 and M30 fragment (two inflammatory related molecules) with anthropometric and metabolic status markers in overweight/obesity subjects under energy restriction, using measurements at baseline and at the end of the intervention (6 months). As expected, the metabolic hepatic and inflammatory outcomes improved in most cases. Interestingly, inflammatory markers (FGF-21, M30 and PAI-I) indicated different clinical outcomes. First, FGF-21 was associated with markers of cell apoptosis (M30) and transaminases in our study. This marker has been shown as an independent predictor of NAFLD and inflammatory processes [48] in humans [20, 31, 49]. Other authors have suggested that FGF-21 can be used for early identification of hepatic steatosis [50]. Thus, circulating FGF-21 levels in humans have been found increased in pathologies such us obesity, metabolic syndrome insulin resistance and cardiovascular disease [51, 52]. Also, another study [53] demonstrated that FGF-21 was associated with hs-CRP, whose values were significantly elevated in patients with NAFLD and diabetes. On the other hand, several authors have demonstrated the relation of insulin resistance and diabetes with FGF-21, as occurred in our analysis [40]. In other words, FGF-21 has been related with processes of inflammation in NAFLD [54] and FGF-21 could have a compensatory effect to protect the body from adverse metabolic responses. The findings in human studies suggest that serum FGF-21 level has the potential to be an important biomarker for the early diagnosis of metabolic diseases or its complications [51]. Secondly, a study from Kawanaka et al. concluded that serum CK-18 (M30 fragment) levels can predict values of NASH in NAFLD patients [50]. Furthermore, it has been reported that CK18, including M30 and M65 fragments, is predictive of the prognosis of NAFLD [55]. However, another study suggests that plasma CK-18 has a high specificity for NAFLD and fibrosis, but its limited sensitivity makes it inadequate as a screening test for staging NASH [56]. Thus, it has been suggested that combined with other biomarkers such as FGF21, CK18 might be a complementary marker in non-invasive identification of NAFLD patients [50]. Third, PAI-I metabolism and functions are still scarce, although some studies have suggested that these cytokines could have a later effect in the obese state [57]. In a study from Thiruvagounder et al. increased levels of PAI-I showed significant associations with NAFLD and impaired fibrinolytic activity [58]. Contrarywise, in our study PAI-I showed a significant reduction at the end of the intervention and PAI-I revealed significant positive associations with M30 fragment, GGT and FGF-21. Finally, according to the regression analysis in this study, it was found that when adjusted for changes in weight loss, the inflammatory markers are not affected. A limitation of this study is that NAFLD was evaluated using non-invasive markers instead of imaging techniques and/or liver biopsies. Also, the sample size of the current study is relatively small, and conclusions should not be extrapolated to the general population. Although type I error cannot be excluded, the obtained results reveal that the outcomes are concordant with the adopted hypothesis. Moreover, the design of the current trial is based on validated non-invasive and affordable markers, which makes them a suitable form of diagnosis in clinical practice. In addition, FLI was assessed in subjects at baseline and the whole sample presented moderate (FLI ≥ 30 and ≤ 60) and severe (FLI > 60) fatty liver. One of the main strengths of the present research is that it is a randomized controlled trial, considered the gold standard in the hierarchy of research designs for evaluating the efficacy and safety of a treatment intervention. Furthermore, the fact that every dietary pattern has been personally designed for each patient taking into account the sex, height, initial body weight and physical activity should also be highlighted. Finally, it is important to point out that a well-recognized healthy dietary pattern (AHA) was used as a comparative test, which shows that positive results obtained with the RESMENA diet should be considered of reasonable importance. As a corollary, the current research indicates that FGF-21 is sensitive to nutritional stress regardless of weight lowering, and that the mechanism of inflammation can be independent of weight loss.
In conclusion, the current study demonstrates that FGF-21 levels are modulated after an energy-restriction treatment in metabolic syndrome-obese patients concomitantly with an improvement in inflammation, hepatic damage and body composition. Importantly, the FGF-21 changes exhibited a strong association with M30 fragment and PAI-1, two non-invasive markers of liver inflammation. This association is independent of weight loss. Therefore, further investigation of FGF-21 is required since this molecule appears to be involved in the obesity-inflammation-liver process, and its plasma concentrations could add relevant information in the prediction and management of the liver pathogenesis related to obesity.