Introduction
Enterococcus faecalis and E. faecium can cause community-acquired and nosocomial infections. In recent decades, an increase in the occurrence of vancomycin-resistant enterococci (VRE) has been observed in Europe, with E. faecium being the most dominant species [1–3]. They have been isolated frequently worldwide and are difficult to treat [4–7].
Because enterococci have intrinsic resistance to some classes of commonly used antibiotics and the ability to acquire resistance to most of the current available antibiotics, either by mutation or by receipt of foreign genetic material, infections caused with multidrug-resistant enterococci are particularly difficult to treat [5, 8].
Although new antimicrobial agents designed to treat infections caused by multidrug-resistant pathogens have been introduced in the past few years, there has been a worldwide increase in the incidence of infections caused by VRE [4, 6, 7, 9]. Other choices for overcoming drug resistance include synergistic combinations of antimicrobials. Combination antibiotherapies have a distinct advantage over monotherapies in terms of their broad spectrum and synergistic effect at lower doses. They are sometimes used in an attempt to prevent or delay the in vivo emergence of drug-resistant subpopulations of pathogenic organisms [10, 11]. Linezolid is the first member of the structurally novel and totally synthetic antibiotic group named oxazolidinones, which acts by blocking protein synthesis at the ribosome. It was approved by the U.S. Food and Drug Administration in 2000. However, with the excessive use of linezolid during clinical trials and therapy, development of resistant isolates of Enterococcus spp. occurred [12–14].
Serious infections associated with enterococci are usually treated with a combination of penicillin/ampicillin with an aminoglycoside. The emergence of high-level resistance to aminoglycoside in enterococci, especially E. faecium and E. faecalis, seriously affected the therapeutic approach. Vancomycin is an agent acting on the cell wall. Because of the lack of reliable synergistic interaction between a cell wall active antibiotic and an aminoglycoside against high-level aminoglycoside-resistant (HLAR) Enterococcus strains, vancomycin became a first-line drug effective against these strains [15]. The options of therapy of infections caused by Enterococcus spp., which have resistance both to aminoglycosides and vancomycin, have been limited.
In the present study we aimed to investigate the in vitro activity of vancomycin combined with linezolid against VRE strains with high-level aminoglycoside resistance.
Material and methods
A total of 30 randomly selected clinical VRE strains were studied. Fourteen out of 30 strains were isolated from blood, and 16 from urine from different patients who were admitted to different clinics of the university’s hospital.
Bacterial identifications of the strains were undertaken using conventional methods. They were identified as the genus Enterococcus if they had the following properties: Gram-positive; catalase negative; ability to grow in 6.5% sodium chloride and 40% bile; hydrolysed esculin; and positive results in pyrrolidonyl arylamidase tests (PYR; BD; USA). The Enterococcus species were identified using biochemical and physiological tests such as arginine dihydrolase, hippurate hydrolysis, growth in pyruvate, pigment production, motility, arabinose, and lactose utilisation, and other carbohydrate utilisation tests by using both a commercial identification system for enterococci (Microgen Strep ID, Microgen Bioproducts Ltd, UK) and inhouse products [15]. All strains were also tested for susceptibilities to ampicillin (10 μg: Oxoid, UK), imipenem (10 μg: BBLTM, USA), and Quinupristin/Dalfopristin (Q/D) (15 μg: Oxoid, UK) using a disk diffusion test. E. faecium strains were resistant to ampicillin and imipenem and susceptible to Q/D, and E. faecalis strains had opposite results [4, 7, 16, 17]. Beta-lactamase enzyme production was also investigated by nitrocefin discs (BD BBLTM, Cefinase, USA). The high-level resistance of aminoglycoside among VRE strains was investigated using 120 μg gentamicin and 300 μg streptomycin (BD BBLTM BENEX Ltd., Ireland) disks [18].
The antibiotics tested in the study were vancomycin (Multicell, USA) and Linezolid (Pfizer Inc., Groton, CT, USA). Teicoplanin (Glentham Life Sciences Ltd., UK) was also studied for phenotyping of the VRE strains. Susceptibility to agents against the strains tested was investigated using broth microdilution assay as described by the Clinical and Laboratory Standards Institute (CLSI) [18, 19]. They were prepared in accordance with the proposals of CLSI and the manufacturers. In all tests, cation-adjusted Mueller-Hinton II Broth (CAMHB) (BBLTM, Becton, Dickinson and Company, France) were used for all experiments. The inoculum of each strain was adjusted to achieve a final inoculum of 105–106 CFU/ml in the wells of the plate. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of antibiotic giving complete inhibition of visible growth, and was interpreted in accordance with the guidelines of the standards for antimicrobial susceptibility testing. Quality-control testing procedures were performed by also testing Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212 as reference strains in each run [18–20].
In vitro activities of antibiotics in combination were assessed using a broth microcheckerboard [11]. The concentrations of antibiotics in combinations were based on two dilutions above and four dilutions below the MICs. The fractional inhibitory concentration (FIC) indexes (FICI) were calculated using the following formula: FICI = FICA + FICB. The FICI was interpreted as follows: synergism, FICI ≤ 0.5; additive/indifference, FICI ≤ 0.5 – ≤ 4; antagonism, FICI > 4 [21].
Results
Twenty-eight of 30 VRE strains were identified as E. faecium and two as E. faecalis, depending on conventional methods and antimicrobial results. All strains were found to be resistant to vancomycin and teicoplanin, and susceptible to linezolid by broth microdilution method. None of the strains detected beta-lactamase enzyme. The MIC values of antimicrobial agents and susceptibility rates are shown in Table I. The MIC50,90 and MICrange values were found as 2, 2, and 2–4 for linezolid, 512, 512, and 512–1024 for vancomycin, and 64, 128, and 16–128 μg/ml for teicoplanin. All strains had the VanA phenotype of glycopeptide resistance [15].
Table I
The minimum inhibitory concentration (MIC) values of antimicrobial agents and susceptibility rates
| Agent | MIC values [μg/ml] | Susceptibility, n (%) | ||
|---|---|---|---|---|
| MIC50 | MIC90 | MICrange | ||
| LNZ | 2 | 2 | 2–4 | 30 (100) |
| VAN | 512 | 512 | 512–1024 | 0 |
| TEC | 64 | 128 | 16–128 | 0 |
[i] LNZ – linezolid, VAN – vancomycin, TEC – teicoplanin. Susceptibility breakpoints: Lnz ≤ 4, Van ≤ 4, Teic ≤ 2 μg/ml [20].
In this study, 24 (80%) of 30 VRE strains were identified as HLAR, five as high-level streptomycin resistant (HLSR), and one strain as non-HLAR. The rate of synergistic effect (FICI: ≤ 0.5) of vancomycin combined with linezolid against 30 VRE strains was found to be 46.6% (14/30) (Table II). One out of the 14 synergistic reactions belonged to the HLSR VRE strain, which was isolated from urine, and 13 to the HLAR VRE strains, which were isolated from both blood and urine samples. All synergistic reactions occurred against E. faecium strains. The rate of the additive/indifference effect (FICI: > 0.5–4) was found to be 53.4% (16/30). Two of them were E. faecalis that were isolated from urine samples. No antagonism was observed (Table III).
Table II
The distribution of fractional inhibitory concentration indexes (FICI) values and interpreted FICI results of the combination against 30 VRE strains
| Combination | Distribution of FICI values (n = 30) | Interpreted FICI results, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.3 | 0.4 | > 0.5 | 0.6 | 0.7 | 2 | Syn | Add/Ind | Ant | |
| VAN + LNZ | 3 | 11 | – | 9 | 5 | 1 | 1 | 14 (46.6) | 16 (53.4) | 0 |
Table III
Distribution of aminoglycoside resistances and combination interactions by species in 30 VRE strains
| VRE (n = 30) | HLAR | Non-HLAR | HLSR | Syn | Add/Int | Ant |
|---|---|---|---|---|---|---|
| Enterococcus faecium (n = 28) | 24 | 0 | 4 | 14 | 14 | 0 |
| Enterococcus faecalis (n = 2) | 0 | 1 | 1 | 0 | 2 | 0 |
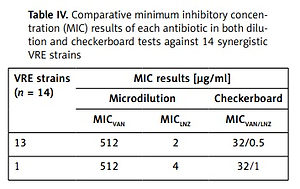
The MIC value distributions of each antimicrobial alone and in combination against 14 synergistic VRE strains are shown in Table IV. The MIC values of each antimicrobial alone against 14 strains given synergistic result were found as 512 μg/ml for vancomycin and 2, and 4 μg/ml for linezolid in microdilution method. However, in a combination of these antibiotics, the MIC concentration of each antibiotic was found as 32 μg/ml for vancomycin and 0.5 μg/ml for linezolid in 13 strains (32/0.5), and 32 μg/ml and 1 μg/ml in one strain (32/1) in the checkerboard method, respectively. The one strain had the HLAR.
Discussion
Linezolid is one of the last-resort antibiotics for the treatment of infections with VRE [17]. However, the increasing prevalence of linezolid resistance among clinical enterococ strains has been reported, especially during treatment of infections [4, 22, 23]. Additionally, resistance to antibiotics that have been used to treat infections caused by VRE, such as tigecycline and daptomycin, has already been reported [4, 6].
Vancomycin is a bactericidal antimicrobial agent that is mainly active against Gram-positive cocci. Although vancomycin has been successfully used in therapy of Gram-positive bacterial infections for years, resistance has been increasing in recent years [4]. Because of the lack of reliable penicillin-aminoglycoside synergism among high-level aminoglycoside-resistant enterococci, vancomycin became a first-line drug effective against enterococci until the time when Enterococcus species–resistant to vancomycin were reported with increasing frequency [7, 15]. The synergistic effect of aminoglycosides and glycopeptide or beta-lactam antimicrobials is lost if there is high-level resistance to aminoglycosides [24]. Infections caused by Enterococcus spp. that have resistance both aminoglycosides and vancomycin have limited therapy options. Hence, it is important to introduce a new alternative method of treatment.
A high rate of resistance to antimicrobials in Enterococcus strains is obviously problematic, and a novel policy is needed to challenge the resistance in these microorganisms [25]. Additionally, the VRE strains with HLAR or HLSR that have aminoglycoside resistance have decreased the combination therapy alternatives to treat the infections caused.
In this study, 24 out of 30 VRE strains were found to have high-level aminoglycoside resistance (24/30). All these strains were E. faecium, which has high rate of resistance to antimicrobials [25]. The vancomycin concentrations alone in combination were found to be 32 mg/l in checkerboard test results (Table III). This concentration is reachable for vancomycin in human serum because it is inform that serum peak levels are reach to 30–40 mg/l in the administration of it at the treatment doses [13].
In conclusion, because of the synergistic results and lack of antagonism, the combination of vancomycin with linezolid can make an important contribution to the treatment of infections caused by VRE strains, especially VR–E. faecium with HLAR, which have limited numbers of alternative treatment choices if more in vitro experiments and in vivo applications on this combination are proven. Additionally, antibiotic combinations that have synergistic interaction have been used to treat infections in an attempt to prevent or delay resistant bacteria from arising.