Introduction
High estrogen levels could reduce pregnancy rates by disrupting the implantation of the embryo into the endometrium in patients treated with fresh cycles of in vitro fertilization (IVF) [1, 2].
Frozen embryo transfer is currently preferred to prevent the negative effects of high estrogen levels in IVF cycle induction on the endometrium, prevent IVF complications such as ovarian hyperstimulation syndrome (OHSS), and increase cumulative pregnancy rates. However, there may be elevated estrogen levels in frozen cycles [3–5]. Estrogen elevation negatively affects endometrial receptivity. It decreases uterine blood flow and prevents implantation and trophoblast invasion [6, 7]. Preeclampsia and intrauterine growth restriction are frequently observed in pregnancies with high estrogen levels [8, 9]. Estrogen has a direct effect on implantation; nevertheless, there are limited studies on the effect of estrogen levels on frozen embryo transfer. Instead of hormonal monitoring, endometrial thickness is typically measured. In routine practice, hormonal monitoring is not performed to assess the effects of estrogen. In frozen embryo transfer, the endometrium is prepared in the form of natural cycles or artificial cycles. In natural cycles, the patient’s own ovulation cycle is monitored, and artificial cycles are preferred as it may be difficult to ensure patient compliance in centers with a high number of IVF cycles. When the endometrium is prepared, gonadotropin-releasing hormone (GnRH) agonists can be used for cycle suppression in artificial cycles or only estrogen and progesterone can be given externally without GnRH agonists. Some studies have reported that there is no difference between the pregnancy rates in cycles with and without GnRH agonists [10, 11]. In our clinic, the preparation of the endometrium with hormone replacement therapy (HRT) is preferred. In the present study, the effect of estrogen on the pregnancy and abortion rates in autologous frozen embryo transfer with HRT was investigated.
Material and methods
The study protocol was approved by the Ethical Committee of the Medical Faculty of Ondokuz Mayıs University (No: OMU-KAEK 2018/475). All frozen autologous embryos transferred from January 2016 to January 2018 at the IVF center of Ondokuz Mayıs University were retrospectively screened.
Inclusion criteria: Only patients with endometrial HRT and day 5 embryo transfer were included in the study. All patients had frozen embryos from a previous IVF cycle. A total of 204 patients aged 18–40 were included in the study.
Exclusion criteria: Patients with a history of more than 3 failed transfers and with an endometrial thickness of less than 7 mm on day 11 were excluded from the study. Patients older than 40 years old and younger than 18 were excluded from the study.
A total of 204 patients aged 18–40 were included in the study. Four patients with endometrial thickness less than 7 mm on day 11 were excluded from the study.
Endometrial preparation was initiated on day 2–3 of the cycle following transvaginal ultrasound with oral estradiol hemihydrate (2 mg Estrofem; Novo Nordisk, Bagsvaerd, Denmark). The endometrial preparation protocol began with 4 mg/day Estrofem on days 1–4, 6 mg/day Estrofem on days 5–8, and 8 mg/day Estrofem from day 9 onwards. A second transvaginal ultrasound was performed after 10 days of estrogen treatment. Embryo transfer was scheduled if the endometrial thickness was at least 7 mm. Progesterone was administered intramuscularly (50 mg Progestan; Koçak, Istanbul, Turkey) at a dose of 100 mg for 5 days prior to embryo transfer (day of progesterone). One or two embryos were transferred depending on the patient’s age and the quality and number of embryos. All of them were 5-day embryos. All transfers were performed without anesthesia using ultrasonography by the same reproductive endocrinologist.
Serum samples were taken to determine estrogen levels on day 2 or 3 of the cycle and on the day of progesterone (when progesterone treatment was initiated). The level on day 2 or 3 of the cycle was indicated as e1, and the level on the day of progesterone was indicated as e2.
Human chorionic gonadotropin (β-hCG) positivity, which was examined on day 14 after the transfer, was used to evaluate biochemical pregnancy. Abortion was defined as the termination of pregnancy before the 20th gestational week.
Progesterone was given intramuscularly (Progestan 50 mg; Koçak, Turkey) and estrogen (Estrofem 2 mg; Novo Nordisk, Denmark) was given orally as luteal support until 12 weeks of pregnancy.
Results
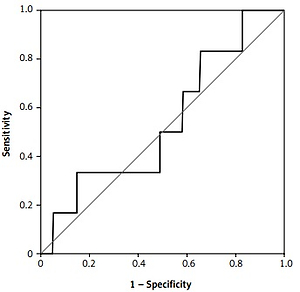
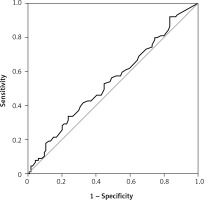
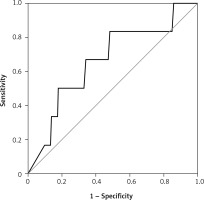
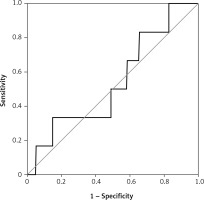
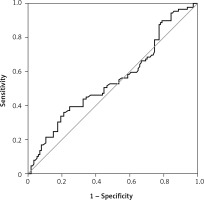
There were 130 patients with unexplained infertility, 20 patients with poor ovarian reserve, and 54 patients with the male factor. In the unexplained infertility group, 58 of the patients were pregnant, and 72 of them were not pregnant. In the poor ovarian reserve group, 4 of the patients were pregnant, and 16 of them were not pregnant. In the male factor group, there were 27 pregnant and 27 non-pregnant patients (p = 0.761). The follicle-stimulating hormone (FSH) level on day 2–3 was 8.348 ±2.080 IU/ml in the pregnant group and 7.179 ±2.919 IU/ml in the non-pregnant group (p = 0.0809). The antral follicle count was 16.09 ±3.988 in the pregnant group and 14.36 ±5.144 in the non-pregnant group (p = 0.2793). The average patient age was 30.65 ± 4.996 in the pregnant group and 31.21 ±4.654 in the non-pregnant group (p = 0.5680). The endometrial thickness on transfer day was 10.22 ±1.536 mm in the pregnant group and 9.643 ±1.311 mm in the non-pregnant group (p = 0.1606) (Table I). The median e1 level was 38 pg/ml in the pregnant group and 35 pg/ml in the non-pregnant group (Figure 1). There was no statistically significant difference (p = 0.273). Similarly, there was no statistically significant difference between the e2 levels (Figure 2) of the pregnant and non-pregnant patients (p = 0.219). The median level was 208.5 pg/ml for non-pregnant women and 202 pg/ml for pregnant women (Table II). The median e1 level was 21.5 pg/ml for patients who had an abortion and 37 pg/ml for those who did not have an abortion, which did not show a statistically significant difference (p = 0.117) (Figure 3). Similarly, there was no statistically significant difference between e2 levels according to the abortion rate (p = 0.722) (Figure 4). The median level was 205 pg/ml for patients who did not have an abortion and 216.5 pg/ml for patients who had an abortion (Table III).
Table I
Demographic details
Table II
Comparison of e1 and e2 values according to pregnancy status
| Parameter | Negative | Positive |
|---|---|---|
| e1 [pg/ml] | 35.0 (5–153) | 208.5 (45–638) |
| e2 [pg/ml] | 38.0 (5–138) | 202.0 (77–498) |
| P-value | 0.273 | 0.219 |
Table III
Comparison of e1 and e2 values according to occurrence of abortion
| Parameter | Women who had an abortion | Women who did not have an abortion |
|---|---|---|
| e1 [pg/ml] | 37 (5–153) | 205 (45–638) |
| e2 [pg/ml] | 21.5 (5–60) | 216.5 (99–308) |
| P-value | 0.174 | 0.722 |
Figure 2
Receiver operating characteristic curve curve of the e2 value according to the pregnancy status
Discussion
In a normal menstrual cycle, progesterone and estrogen in the endometrium in the follicular phase are required for endometrium maturation [12]. However, estrogen should increase within a certain limit as high estrogen levels may impair implantation [3]. High estrogen levels could reduce uterine vascularization, inhibit the invasion of trophoblasts, and suppress the expression of genes needed for implantation [13]. Estrogen levels in natural cycles gradually increase and remain above 200 pg/ml for at least 50 h, and an luteinizing hormone (LH) surge is triggered. Subsequently, estrogen levels decrease after reaching 300–400 pg/ml in the ovulation phase. We mimicked this natural cycle by using a step-up regimen in HRT cycles in our clinic; however, the estrogen decrease after ovulation in natural cycles was not observed in HRT cycles, and estrogen levels in the follicular phase were occasionally above physiological levels. It has been suggested that high estrogen levels in HRT cycles before the day of progesterone may inhibit implantation by disrupting embryo-endometrium synchronization. Niu et al. demonstrated that e2 levels on the progesterone day in frozen embryo transfer with HRT did not affect the results of the pregnancy or implantation rate [5]. Banz et al. reported that estrogen levels did not affect the results of pregnancy when the endometrium was 7–15 mm in frozen HRT cycles with estrogen patch and vaginal progesterone [14]. Remohi et al. conducted a study of oocyte donation cycles using oral estrogen after GnRH agonist induction and reported that there was no relationship between the estrogen levels on the progesterone day and the implantation or pregnancy rate [15]. Similar to our study, various studies have found that estrogen levels do not affect the results of pregnancy [16–18]. Estrogen and progesterone are necessary for the preparation of the endometrium for pregnancy. However, even very low levels of estrogen do not reduce pregnancy rates [15]. Consistently, the present study and other studies indicated that high estrogen levels would not have a negative effect on the results of pregnancy. For pregnancy there is a wide range of estrogen levels. This suggests that estrogen exerts an effect on the endometrium through cytokines and adhesion molecules [18]. Therefore, changes in estrogen levels would not affect implantation. Interestingly, Fritz et al. reported that the maximum estrogen levels were not different between pregnant and non-pregnant women; however, patients with lower e2 levels had higher rates of pregnancy. Unlike our study, estrogen levels were examined four times in each cycle in the study by Fritz et al. In addition, the study used intramuscular and transdermal e2. On the other hand, in our study, oral estrogen was used. Oral estrogen is a weaker estrogen, which is transformed into estrone during the first transition in the liver and intestine. This first transition effect is reduced for intramuscular and transdermal estrogens [12]. However, although Banz et al. used transdermal estrogen in their study, their results were similar to ours. A limitation of the present study is that it is a retrospective study with a first-pass effect. However, all patients followed the same protocol, which is a strength of the study. The results of our study demonstrated that estrogen levels in frozen embryo transfer with HRT did not affect pregnancy results and that estrogen monitoring was not necessary. Further prospective studies are needed to confirm the findings.
There are limited studies about the effect of estrogen levels on frozen embryo transfer, and this study is important because we found that it is not necessary to monitor estradiol levels in HRT cycles.