Acute lymphoblastic leukemia (ALL) is the most common cancer in children, adolescents, and young adults and remains the leading cause of cancer related death among the pediatric group of patients [1]. Thus finding early predictive biomarkers is still important to detect children with poor outcomes, for whom quickly altered treatment plans are vital.
Gamma-delta (γδ) T cells are a recent topic of growing interest in the field of prediction of a favorable outcome in numerous malignancies. Most of the studies have shown that the amount of γδ T cells infiltrating solid tumors is a strong biomarker of good prognosis for cancer patients [2, 3]. On the other hand, there are single reports demonstrating their pro-tumor role and strong correlation with advanced tumor stages, lymph node metastasis or poor outcome [4].
To our knowledge, no data have been reported to date on γδ T cell counts among pediatric patients with acute leukemia at diagnosis. Similarly, no evidence has been presented previously about lymphocytes and their correlation with already known risk factors in childhood ALL. Due to these facts, we decided to evaluate these associations to verify the potential use of selected subsets of γδ T cells (CD3+γδ , CD4+γδ, CD8+γδ) as a novel and early biomarker of childhood leukemia severity.
This study was approved by the local research ethics committee. All samples were obtained under written informed consent. Nineteen patients (9 male and 10 female) aged 2 to 18 years were recruited to the study. Diagnosis of acute lymphoblastic B-cell leukemia was verified via cytological, cytochemical, and immunophenotyping methods. Blood samples were collected before induction following protocol IA according to ALL IC BFM 2009. In one of the patients BCR/ABL mutation was detected. At the end of the protocol, 13 of the patients were classified in the intermediate risk (IR) group, 5 in the high risk (HR) group, and 1 patient in the standard risk (SR) group. Data regarding hematological parameters were obtained from medical records. Due to the small group of patients, we could not analyze correlations between γδ T cells and cytogenetic, immunophenotypic, and central nervous system (CNS) status.
The control group consisted of 19 healthy people, aged 2–18 years (11 male and 8 female). Children with active infection, acute/chronic diseases, receiving immunosuppressive therapy or with oncological history were excluded.
Freshly obtained EDTA whole blood was stained using antibody cocktails as follows: TCRγδ FITC (clone, IMMU510)/TCRαβ PE (clone, IP26A)/CD4 APC (clone, 13B8.2)/CD8 AF700 (clone, B9.11)/CD3 Krome Orange (clone, UCHT1). Samples were then lysed with the Immunoprep Reagent Kit and TQPrep Workstation, measured with a Navios flow cytometer, and analyzed with Kaluza software (all from Beckman Coulter, USA). For proper gating fluorescence the minus one approach was used. First, lymphocytes were gated according to FSc/SSc, and then CD3+/CD4+ and CD3+/CD8+ T lymphocytes were identified. Finally, for both T CD4+ and T CD8+ lymphocytes, the percentage of TCRγδ+/TCRαβ– was noted.
Statistical analysis was performed using IBM SPSS 25. Depending on the distribution of the variable, the nonparametric Mann-Whitney U test, Wilcoxon test, or parametric Student’s t-test was used to compare the differences between the normal control and leukemic patient group as well as differences among study groups. The correlation between γδ and prognostic factors was analyzed by correlation coefficients (Spearman’s, Pearson’s). A value of p < 0.05 indicated statistical significance.
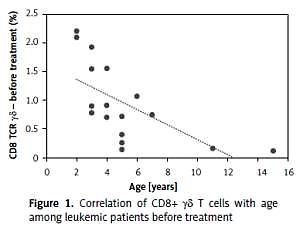
We evaluated the association of γδ T cells with a series of clinical and hematological agents. Interestingly, we observed differences in the percentage of CD8+ γδ depending on age in leukemic patients (R = –0.67, p < 0.00) (Figure 1), but not in healthy controls (p = 0.58). The same investigation showed no statistically significant differences in the percentages of CD3+ γδ and CD4+ γδ lymphocytes between leukemic patients and healthy controls.
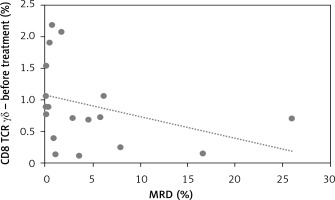
We did observe a significant negative correlation between CD8+ γδ T cells at presentation with a crucial prognostic factor in childhood ALL: minimal residual disease R = –0.53, p = 0.018 (Figure 2).
The same investigation showed no significant differences between CD3+ and CD4+ γδ lymphocytes and the same risk factors.
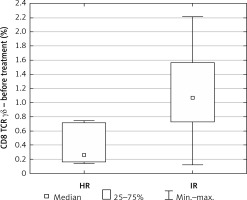
Due to only 1 patient being classified in the standard risk group, he was excluded from the evaluation.
The percentage of CD8+ γδ T cells at diagnosis was definitely higher in the IR group relative to the HR group (median IR = 1.07 vs. median HR = 0.26, p = 0.03) (Figure 3).
Gamma-delta (γδ) T cells are a topic of growing interest due to their prognostic signature of favorable outcome in numerous cancers [2]. To our knowledge, no data have been reported to date on γδ T cell counts among pediatric patients with acute leukemia at diagnosis. Similarly, no evidence has been presented previously about lymphocytes and their correlation with already known risk factors in childhood ALL. Thus far, γδ T cell counts have been examined in children with ALL following hematopoietic stem cell transplantation (HSCT). The results of the study argued in support of a favorable prognostic role for γδ T cells. γδ T cells have been found with increased frequency in patients who proceed to become free of disease after HSCT compared to patients with normal or decreased numbers of γδ T cells [5].
In our study we observed a higher percentage of CD8+ γδ T cells in younger patients. This correlation was not observed in the control group. Taking into account the fact that the explanation for the better outcome during the age of 1–6 and worse during adolescence is still unclear, it could be a good introduction to further research on lymphocytes’ involvement in the age-related response to leukemia treatment [6]. Apart from that, we found a strong negative correlation between CD8+ γδ T cells and minimal residual disease (MRD), which is a key prognostic factor reflecting the number of lymphoblasts in bone marrow on day 15 of treatment. The amount of CD8+ γδ T cells in peripheral blood was increased in those who achieved a lower percentage of bone marrow blasts on day 15 of chemotherapy. Additionally, an interesting finding was definitely the higher percentage of CD8+ γδ T cells in the group of patients allocated to the IR group relative to the HR group.
In conclusion, our data showed a higher percentage of CD8+ γδ T lymphocytes in patients with favorable prognostic factors. We suppose that circulating CD8+ γδ T cells may act as a novel early biomarker of good prognosis in childhood ALL in future.