Introduction
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism, is one of the leading vascular diseases worldwide, ranking third after cardiovascular and cerebrovascular diseases [1, 2]. The incidence rate of VTE is estimated to be 1/1000 based on research abroad [3, 4]. However, there are limited epidemiological data specific to China [5, 6]. DVT is caused by abnormal blood coagulation in deep veins due to factors such as vascular endothelial injury, slow blood flow, and a hypercoagulable blood state [7]. In its early stages, lower limb DVT often causes swelling and pain in the affected limb [8]. However, severe cases can lead to limb necrosis, pulmonary embolism and other complications. Repeated infection, swelling and ulcer can also occur, significantly impacting the quality of life of patients [9, 10].
Chinese scholars have summarized the treatment and development of DVT as focusing on symptoms, preventing spread, thrombus clearance and addressing the underlying cause [11–13]. Anticoagulant therapy is considered the foundation of DVT treatment; however, its effect is limited. A study by Kahn et al. [14] found that 20–50% of patients who received simple anticoagulant therapy for DVT developed post-thrombotic syndrome (PTS) within a 5-year follow-up period, with a 5–10% incidence of venous stasis ulcer, which significantly impacted their quality life [7].
Anticoagulation therapy remains the cornerstone of DVT treatment; however, its effectiveness is limited. Mechanical thrombectomy (PMT) has emerged as a promising approach. Studies have reported its advantages over catheter-directed thrombolysis (CDT) in terms of faster clot removal, reduced bleeding risk, and improved patient experience [15, 16]. PMT can reduce the thrombus load and shorten the catheterization time, which in turn minimizes bleeding risk, shortens hospital stay, improves the medical experience of patients, and reduces the risk of catheter infection to a certain extent [17, 18]. Garcia et al. reported that the treatment time of 73% of patients with PMT was less than 24 h, and in 36% of patients it could even be shortened to 6 h. Based on the consensus of experts at home and abroad, for patients with acute DVT, PMT can quickly restore blood flow, save valve function, prevent pulmonary embolism and reduce the incidence of postoperative PTS [19].
Due to the unique anatomical structure of the iliac vein, the iliac vein stent is required to have good flexibility and support [20]. At the same time, to enable accurate positioning of the operator during the operation, the stent also needs to have good radiopacity. Iliac vein stenting is generally centered on the lesion, and the primary goal is to fully cover the lesion [21]. With the scouring effect of blood flow, the stent may shift and may not completely cover the lesion range. However, if the stent enters the inferior vena cava too long, it will cause complications such as unstable stent, affecting the contralateral blood flow, thrombus recurrence, inferior vena cava obstruction, and so on. The CIRSE guidelines suggest the use of laser-engraving stents for lesions at the transition of the iliac inferior vein and inferior vena cava, with the stent positioned no more than 10 mm into the latter [17]. Chinese experts agree that the rate of shortening should be taken into account when selecting braided stents, with the head end suggested to be positioned 10 mm into the inferior vena cava [22]. When selecting the laser engraving bracket, the head end enters 3–4 mm into the inferior vena cava [23]. Therefore, on the premise of ensuring complete coverage of the lesion, after fully evaluating the characteristics of the stent, the less the stent enters the inferior vena cava, the better.
Currently, treatment options for lower extremity DVT primarily involve thrombus aspiration or balloon dilatation. However, some patients may have concomitant iliac vein stenosis requiring stenting [24, 25]. The primary aim of this study was to evaluate the efficacy and safety of stent implantation in treating lower extremity DVT with severe iliac vein stenosis, as assessed by thrombolysis rate, DVT recurrence rate, deep vein patency, and clinical outcomes (CIVIQ and CEAP scores).
Material and methods
Clinical data
All procedures and protocols of the study were approved by the local Medical Ethics Committee of Changxing People’s Hospital, Zhejiang, China (Ethics code No: 2022-045; date of approval: 09/16/2022), which were in complete accordance with the ethical standards and regulations of the studies on human beings set by the Declaration of Helsinki (2014).
At the start of the study, the aim and objectives of the study along with possible risk and benefits to the patients were clearly explained to the patients. All patients signed a written informed consent form for participating in the study. This was an observational clinical study conducted on patients with lower extremity DVT complicated with iliac vein stenosis and/or occlusion who received either stent implantation or thrombus aspiration. The patients with stent implantation were assigned to the experimental group, and the patients without stent implantation were assigned to the control group.
The sample size was calculated based on a power analysis with a significance level of 0.05 and a power of 80%, assuming a 20% difference in the primary outcome (thrombolysis rate) between the two groups [19].
The inclusion criteria for our study required that patients have a confirmed diagnosis of lower extremity DVT accompanied by severe iliac vein stenosis or occlusion, as validated through Doppler ultrasound or venography. Eligible participants were required to be aged between 45 and 80 years and present with clinical symptoms such as limb swelling, pain, or discoloration indicative of venous insufficiency. Furthermore, all patients provided written informed consent prior to participation. According to the exclusion criteria, individuals with active bleeding disorders or those currently receiving anticoagulation therapy deemed unsuitable for our study protocol were excluded. Also, patients who had undergone major surgical procedures within the preceding month were excluded, as were those with significant comorbidities, including severe cardiovascular, hepatic, or renal dysfunction, which could potentially complicate treatment outcomes. Additionally, female patients who were pregnant or breastfeeding were not included in the study.
Sixty patients with lower extremity DVT with iliac vein stenosis and/or occlusion admitted to Changxing People’s Hospital, Zhejiang, China between December 2017 and March 2022 were enrolled in this observational clinical study. Patients were divided into two groups: the stent implantation group (n = 14) and the control group (n = 46). There were 32 males and 28 females. The age ranged from 46 to 87 years, with a mean of 68.9 ±12.01 years. The course of disease ranged from 0.5 to 14 days, with a mean of 7.83 ±4.56 days. There were 4 cases of central type and 1 case of mixed type. There were 42 cases involving the left lower limb and 18 cases involving the right lower limb. Twelve cases were complicated with femoral abscess; iliac vein compression was found in 32 cases. The comparisons of demographic information and clinical data between the two groups are presented in Table I.
Therapeutic method
Seven patients received mechanical thrombus aspiration, 12 patients underwent mechanical thrombus aspiration and balloon dilatation, and 6 patients underwent mechanical thrombus aspiration, stent implantation and balloon dilatation. Urokinase (600 000 U/D) was continuously pumped through a thrombolytic catheter or sheath, and enoxaparin (4000 U/12 h) was injected subcutaneously to monitor coagulation indexes (when fibrinogen was maintained > 1.0 g/l, the dosage of urokinase was halved and urokinase was stopped when it was less than 1.0 g/l). After thrombolysis, oral warfarin or rivaroxaban was given. The initial dose of the former was 5 mg/day, the coagulation indexes were monitored after 3 days, and the international standardized ratio (INR) was adjusted to 2.0–3.0. The initial dose of the latter was 15 mg (twice per day), which was changed to 20 mg/day after 3 weeks. Forty-eight hours follow-up angiography showed that 16 patients had iliac vein stenosis (diameter > 60%), so 7 patients underwent iliac vein balloon dilatation and stent implantation. After discharge, the patients continued to take warfarin (INR maintained at 2.0–3.0) or rivaroxaban orally for more than 6 months, take Melissa flow extract tablets 400 mg (3 times per day) orally for 6 months, and wear elastic socks for more than 6 months.
Observation index and scoring method
The recent thrombolysis rate, incidence of bleeding complications, detumescence rate before discharge, middle-term deep vein patency rate, DVT recurrence rate, iliac vein occlusion rate, CIVIQ score and clinical manifestation, etiology, anatomy and pathophysiology (CEAP) grade were observed and counted after surgery [26]. The total patency of the lumen was 0; partial patency and occlusion were respectively 1 and 2. Thrombolysis rate = (preoperative score – postoperative score)/preoperative score × 100%, swelling reduction rate = (preoperative limb circumference difference – postoperative limb circumference difference)/preoperative limb circumference difference × 100%. Iliac vein patency > 90% is complete patency, 50–90% is partial patency, and < 50% is not patency.
Statistical analysis
IBM SPSS Statistics for Windows version 22.0 was used to analyze the data. All continuous variables were first assessed for normality with the Kolmogorov-Smirnov normality test. The variables with normal distribution were presented as the mean ± standard deviation (SD), and variables with skewed distribution were presented as the median (interquartile range, IQR). Statistical analysis was performed using appropriate parametric (t-test) and non-parametric (Mann-Whitney U test) tests based on the distribution of the data. For variables without a normal distribution, the nonparametric Kruskal-Wallis H test was used for comparative analyses of the variables between the groups. The pair-wise comparisons between the groups or conditions were performed using the χ2 test.
Results
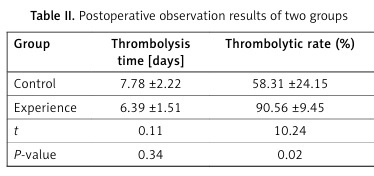
In all 60 cases, the procedures were successfully completed during the study and the data were included in the final analysis. There were 2 cases of bleeding at the puncture point in the stent group, 1 case of subcutaneous ecchymosis at the thigh of the affected limb, and 2 cases of bleeding at the puncture point in the non-stent group. There was no significant decrease in hemoglobin and no obvious hematoma formation. There was no significant difference in thrombolytic time and incidence of bleeding complications between the two groups (p > 0.05), but there was a significant difference in thrombolytic rate and lower limb swelling rate (p < 0.05) (Table II).
Table II
Postoperative observation results of two groups
The mean follow-up was 15.2 ±5.8 months. In the stent group, 1 case developed in-stent DVT 13 months postoperatively, 1 case developed in-stent stenosis 13 months postoperatively, 3 cases recurred in the control group, and in 5 cases iliac vein occlusion occurred. The deep vein patency rate in the stent group was significantly higher than that in the control group (χ2 = 20.23, p = 0.01), the recurrence rate of DVT was lower than that of the control group (χ2 = 1.29, p = 0.34), the iliac vein occlusion rate was significantly lower than that in the control group (χ2 = 18.24, p = 0.01), the CIVIQ score was significantly higher than that of the control group (t = 2.54, p = 0.01), and the CEAP score was significantly lower than that of the control group (t = 3.72, p < 0.01) (Table III).
Table III
Postoperative observation results and comparisons of the parameters between the two groups: control and experimental
Discussion
Thrombolysis is the first choice for patients with lower extremity DVT and iliac vein stenosis, but the traditional anticoagulation is not thorough enough to reduce the incidence of PTS. Due to this, CDT and AngioJet mechanical thrombus are becoming increasingly popular. Previous studies have shown that if the lesions left after iliofemoral vein thrombolysis are not treated, the 2-year thrombus recurrence rate ranges from 47% to 73%. In addition, previous studies on the treatment of lower extremity DVT with iliac vein stenosis mostly did not conduct a multi-index long-term follow-up and compare the medium and long-term efficacy of stent implantation versus non-implantation. This study compared and analyzed the short-term safety and efficacy of iliac vein balloon dilatation stent implantation or conservative treatment after CDT in 60 patients with lower extremity DVT with iliac vein stenosis/occlusion. The study also evaluated the medium-term differences in deep vein patency rate, DVT recurrence rate, iliac vein occlusion rate, CIVIQ score and CEAP grade.
Previous studies have suggested that interventional treatment for iliac vein stenosis is appropriate for patients with typical symptoms of lower limb venous hypertension, such as lower limb swelling, varicose veins, chronic ulcer formation or skin pigmentation [7, 27]. Angiography results showed that iliac vein stenosis was over 60%, and a large number of pelvic collateral vessels were opened. After balloon dilatation, the vascular stenosis remained above 30%, and the pelvic collateral did not disappear or decrease significantly. Therefore, it may be more reasonable to comprehensively consider imaging, symptoms and signs, pressure difference and other factors for quantitative evaluation. Whether to implant stents in iliac vein stenosis is still controversial.
Matsuda et al. reported that among 30 patients with iliac vein compression syndrome, 27 patients with iliac femoral vein stent implantation had a patency rate of 100%, and 3 patients without stent implantation had iliac vein occlusion within 6 months [28]. The ultrasound showed that the stent was unobstructed 6 months later. The iliac vein occlusion rate in the stent group was significantly lower than that in the control group (2.85:48.63, t/χ2 = 17.68, p = 0.01). The deep vein of some patients without stent implantation is still unobstructed, which was considered to be related to thorough thrombolysis, postoperative anticoagulation and the application of elastic socks.
To elucidate the role of stent implantation in the management of lower extremity DVT with severe iliac vein stenosis, it is essential to examine the underlying mechanisms of action, as the deployment of a stent enhances venous patency by mechanically dilating the obstructed [29] segment of the iliac vein, thereby facilitating improved venous return and alleviating symptomatic manifestations such as limb edema and discomfort [16]. In comparative analyses, while anticoagulation therapy remains a cornerstone of DVT management, it does not directly address anatomical obstructions, and thrombolytic therapy, although effective in dissolving thrombi, carries a heightened risk of bleeding complications [30]. Surgical interventions, while beneficial, may involve prolonged recovery and increased morbidity; thus, stent implantation presents a compelling alternative for patients with significant stenosis requiring prompt symptomatic relief [29]. Furthermore, long-term outcomes associated with stent implantation show reduced rates of DVT recurrence and a lower incidence of post-thrombotic syndrome, alongside significant improvements in quality of life measures, including pain relief and functional capacity, underscoring its potential not only as an effective treatment for acute DVT but also as a strategic intervention for enhancing long-term patient outcomes [31].
This study has some limitations that should be considered when interpreting its findings, particularly when generalizing the results to the broader population. The primary limitation is that we only evaluated deep vein patency during mid-term follow-up using angiography, without taking into account other important factors such as lower limb edema, varicose veins, pigmentation, and ulcer formation. Furthermore, the influence of functional reflux resulting from intraoperative deep vein valve damage requires additional investigation. It remains unclear whether the position of the stent and the length of the stent entering the inferior cavity during the operation affect the recurrence of thrombus. While this study provides valuable insights, future studies with larger sample sizes and longer follow-up periods are needed to further validate these findings and assess the long-term impact of stent implantation on patient outcomes. Additionally, the influence of functional reflux after intraoperative deep vein valve damage also needs further investigations.
In conclusion, the comparative analysis of this study shows that the thrombolysis rate, medium-term deep vein patency rate, CIVIQ score and CEAP grade of patients with lower extremity DVT and iliac vein entrapment treated with iliac vein balloon dilatation stent implantation after CDT and/or thrombus aspiration are better than those without stent implantation.