Introduction
Hematopoietic stem cell transplantation (HSCT) is increasingly used as a curative procedure for various hematologic malignancies. HSCT is classified into two types according to the donor, being either allogeneic or autologous [1–3]. Overall survival after HSCT has improved significantly over the past two decades due to improvements in pre-transplantation conditioning protocols and post-transplant immunosuppression. In the light of this increased survival rate, attention must be paid to potential complications associated with the transplantation [4].
HSCT is associated with several comorbidities such as pulmonary complications (PCs), infections, pain, fatigue, muscle weakness, decreased balance function, and graft-versus-host disease (GVHD), which directly affect the functional capacity and quality of life of HSCT patients [5]. The development of these complications may be attributed to the long period of physical inactivity and the direct toxicity from chemotherapy and radiation therapy in patients undergoing HSCT [6–8].
Many factors contribute to the development of PCs in about 40% to 60% of HSCT recipients [9, 10]. These include the underlying disease, pre-transplant induction therapy, the type of HSCT, the time taken for the transplant, and activity restriction. The sedentary lifestyle of HSCT patients even before the transplantation, because of the chemotherapy and radiation therapy they receive, can result in the reduction of respiratory muscular strength (RMS), diaphragmatic excursion, and pulmonary volume, and in the accumulation of secretions in the airways [11–17]. Moreover, the combination of high-dose chemotherapy and total body irradiation (TBI) causes direct pulmonary toxicity and can result in radiation-induced lung injury or interstitial pneumonitis, which increases the incidence and severity of PCs and impairs cell-mediated and humoral immunity for up to 6 months, possibly extending to 12 months [18, 19].
Pre-transplant physical therapy (PT) rehabilitation is essential since patients undergoing HSCT should meet a minimum level of physical performance status to be eligible for HSCT. There is growing evidence that PT is a promising additional pre- and post-HSCT intervention because of its positive multidimensional impacts on muscle strength, fitness, respiratory function, and quality of life [20, 21].
Improving pre-transplantation cardiorespiratory fitness and muscle strength reduces the risks of pneumonia and atelectasis and improves post-transplantation aerobic capacity, especially in individuals with GVHD who require a long-term stay in a protective environment [22–24].
The rehabilitation of HSCT patients is challenging, both physically and emotionally. One of the basic PT goals for the patient after HSCT is to prevent pneumonia and improve pulmonary circulation. Chest physical therapy (CPT) is used to clear secretions, reduce pulmonary infection and improve pulmonary function (PF) [23, 24]. The impact of CPT is not well defined, and therefore the aim of the present study was to evaluate the efficacy of CPT during the pre-transplant phase in patients waiting for HSCT by referring to spirometric values as well as RMS.
Material and methods
Design and sample
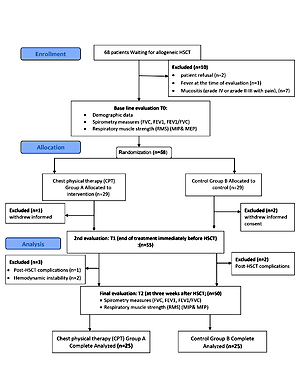
This randomized controlled trial design was conducted on patients scheduled for HSCT at the bone marrow transplant unit within the Cancer Institute. Sixty-eight patients scheduled for HSCT were recruited for the study during the period between June 2018 and December 2019. They were under the care of a hematologist and were waiting for a bone marrow transplant (BMT). Among these, 58 (85%) patients were eligible to participate in the study, and only 50 (73.5%) completed the final evaluation. Before engaging in the study, every patient was asked to sign a consent form. The research was designed following the Declaration of Helsinki criteria. The study proposal was approved by the Ethics Committee of the institution (P.T.REC/012/003367).
After obtaining written informed consent from, patients were scheduled for an appointment to complete baseline measurements (T0) and receive study arm assignment; this was timed to take place 3 weeks before HSCT. All eligible patients were informed about the study during their preparation visit 3 weeks before their admission for allogeneic HSCT. Equal randomization to the CPT (A) and control (B) groups was achieved in the simplest manner, by a manual drawing of numbers on opaque paper. Group A was subjected to CPT, which consisted of postural drainage, diaphragmatic breathing exercises, inspiratory muscle training using incentive spirometry, forced expiration (coughing, huffing), and mobilizing techniques (percussion, vibration, shaking) in addition to routine medical treatment. Group B was subjected to the same routine medical treatment only. Concealed allocation was achieved because the research assistant who carried out the randomization was not involved in any part of the study procedure. The assessment was done for all patients by an investigator who was blinded to the patient’s allocation and had no access to the patient’s information. Patients in both groups received standard physical therapy care during the inpatient waiting period, which focused on keeping patients as active as possible in terms of their daily living activities.
Inclusion criteria
Patients (of both genders) were included in the study if they had been admitted to the HSCT unit, completed the induction therapy and were awaiting allogeneic HSCT. Additional inclusion criteria were: age range from 40 to 55 years, non-alcoholics, medically cleared to exercise by the transplant physician, and able to understand training instructions and follow the study protocol.
Exclusion criteria
Participants were excluded if they had one of the following: fever at the time of assessment, mucositis (grade IV or grade II–III with pain), post-HSCT complications that required hospitalization, or hemodynamic instability (Figure 1).
Outcome measures
Measurements were obtained 3 weeks before HSCT (T0) as a baseline assessment, then at the end of treatment immediately before HSCT (T1) and the last assessment at 3 weeks after HSCT (T2).
Spirometry measures
Spirometry is a device that measures the rate at which the lung changes volume during forced breathing. Evaluations were conducted while the patient was in a sitting position, using a Helios 702 device (portable spirometer, Ambala, Haryana, India), following the guidelines for pulmonary function tests. The spirometry was calibrated on a regular daily basis. A disposable mouthpiece and bactericidal filter were coupled to the spirometer. Measurements with a nose clip were taken three times at 1-minute intervals.
The patient was instructed to inhale as deeply as possible, rest for 1 to 2 s and then exhale with maximum effort. The forced vital capacity (FVC), in which the patient inhales maximally and then exhales as rapidly and as completely as possible, the forced expiratory volume in 1 s (FEV1), which is the volume of air exhaled in the first second of the FVC maneuver, and the relation between FEV1 and FVC were measured [25].
During the spirometric measurements, patient effort and cooperation were required. Visual and verbal encouragement were given to the patients during the assessment. Air leaks, mouthpiece obstruction, and the Valsalva maneuver were avoided during the assessments. Measurement values were expressed in liters and the highest value was considered for analysis.
Respiratory muscle strength
To assess respiratory muscle strength (RMS), maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were measured using a MicroMedical MicroRPM 01 (Respiratory Pressure Meter) according to American Thoracic Society and European Respiratory Society guidelines [26]. The device is a portable battery-operated non-invasive respiratory pressure meter with a clear digital display of the results.
Maximal inspiratory pressure
Maximal inspiratory pressure (MIP) is a composite of the pressure generated by the inspiratory muscles and the elastic recoil pressure of the lungs and chest wall. MIP was determined with empty lungs at residual volume (RV) by measuring upper airway pressure during a maximal voluntary inspiratory effort. Every patient was asked to expire to RV then perform the forceful inspiratory maneuver, sustaining it for 1 to 2 s. The best result from three tests was recorded and used for analysis as the value of inspiratory muscle strength, with a 1- or 2-minute rest and recovery period between efforts.
Maximal expiratory pressure
To measure MEP, every patient was asked to perform a maximal expiratory effort, sustaining it for 1 to 2 s. The best result from three tests was recorded and used for analysis as the value of expiratory muscle strength, with a 1- or 2-minute recovery period between efforts.
Intervention
Chest physical therapy (CPT)
Patients in group A were subjected to a supervised CPT program daily for 3 weeks before HSCT. The program consisted of postural drainage, diaphragmatic breathing exercises, coughing, huffing, percussion, shaking, and exercise training using an incentive spirometer in addition to routine medical treatment. The total duration of each CPT session ranged from 45 to 60 min according to patient tolerance. The choice of airway clearance method that was applied depended on two factors, patient preference and the individual response of the patient to treatment.
Postural drainage
Postural drainage (PD) involves placing the patient in different positions to clear secretions from the lower respiratory tract with the assistance of gravity. PD is effective when there is a large quantity of mucus with low viscosity. The time spent in each position was dependent on the quantity and viscosity of secretions [27].
Diaphragmatic breathing
Every patient in group A was asked to inhale maximally through the nose by directing airflow toward the epigastric region and then expire calmly through the mouth, with vibration applied by the therapist’s hand at the epigastric region at the end of inspiration and throughout the expiration. The frequency of breathing exercises was three series of ten repetitions, once per day [28].
Cough and huff
A cough is a forced expiratory effort, and it is the most important component of the CPT program because it brings secretions present in the larger airways outside the respiratory tract. Huffing is not as forceful as a cough, and therefore the flow and intrapulmonary pressure are much lower compared to coughing. However, as it is done while the glottis is open, huffing can work better and be less tiring to the patient [29].
Chest percussion, vibration, shaking
Chest percussion, vibration, and shaking involve mechanical forces applied to the anterior, lateral, and posterior thorax in order to mobilize thick secretions with a higher viscosity. Chest percussion is the manual application of rhythmic clapping to the thorax cage with a frequency of about 3–6 Hz [30]. Chest vibration is a manual oscillatory movement that is applied during expiration, and chest shaking is a more forceful movement applied to the thorax cage throughout expiration. Each technique was applied for 10 to 20 min according to the patient’s tolerance [31].
Incentive spirometry
Incentive spirometry (IS) is the most widely used method for the prevention and treatment of respiratory problems in postsurgical patients, by maintaining the patency of airways at risk of closure. The patient is asked to take 10 slow maximal inspirations and to hold each breath for as long as possible. This routine is repeated every 2 h throughout the day [32].
Data analysis
Data were collected and analyzed first through exploratory analysis to verify central tendency and dispersion measures and to test data normality. This preliminary investigation aimed to define the statistical tests needed. IBM SPSS Statistics software (version 23.0) was used to analyze the data. Means and standard deviations were calculated, and qualitative data were expressed as percentages and frequencies. To test the normal distribution of the data the Shapiro-Wilk test was applied. Non-paired Student’s t-test was used to compare the variables FEV1, FVC, FEV1/FVC, MIP, and MEP between groups A and B. Repeated measures ANOVA tests followed by post hoc Scheffé tests for pairwise comparisons were used to test the treatment and time effects (T0, T1, T2) on the spirometric values (FEV1, FVC, FEV1/FVC) as well as respiratory muscle strength (MIP and MEP). All the statistical tests used were two-tailed, at a significance level of 5% [33].
Results
Demographic data and baseline clinical characteristics
The two groups were comparable before the beginning of the study in terms of their demographic data and baseline clinical characteristics, since all the initial demographic and clinical measures were similar in both groups. There were no statistically significant differences (p > 0.05) between groups regarding gender distribution, smoking status, indications for SCT, age, height, weight, and BMI. In terms of their baseline clinical characteristics, there were no statistically significant differences between the groups in their spirometric mean values for FEV1, FVC, and FEV1/FVC, as well as respiratory muscle strength MIP and MEP (p > 0.05, Table I).
Table I
Demographic and baseline clinical characteristics in both groups
| Variable | Group A | Group B | P-value |
|---|---|---|---|
| Age [years] | 48.16 ±4.67 | 46.80 ±4.52 | 0.301† |
| Height [cm] | 157.92 ±5.70 | 160.80 ±5.64 | 0.079† |
| Weight [kg] | 71.32 ±11.75 | 76.08 ±11.95 | 0.162† |
| BMI [kg/m2] | 28.76 ±5.50 | 29.45 ±4.55 | 0.634† |
| Sex, n (%): | |||
| Male | 12 (48) | 9 (36) | 0.395† |
| Female | 13 (52) | 16 (64) | |
| Smoking status, n (%): | |||
| Non-smoker | 10 (40) | 13 (52) | 0.677† |
| Ex-smoker | 13 (52) | 8 (32) | |
| Current smoker | 2 (8) | 4 (16) | |
| Indications for SCT, n (%): | |||
| Acute lymphoblastic leukemia | 8 (32) | 10 (40) | 0.811† |
| Acute myeloid leukemia | 5 (20) | 3 (12) | |
| Myelodysplastic syndrome | 3 (12) | 2 (8) | |
| Chronic myeloid leukemia, | 2 (8) | 2 (8) | |
| Non-Hodgkin lymphoma | 1 (4) | 2 (8) | |
| Multiple myeloma | 2 (8) | 3 (12) | |
| Aplastic anemia | 4 (16) | 3 (12) | |
| FEV1_T0 | 2.93 ±0.55 | 2.97 ±0.41 | 0.773† |
| FVC_ T0 | 4.17 ±0.46 | 4.12 ±0.53 | 0.735† |
| FEV1/FVC_T0 | 0.71 ±0.15 | 0.73 ±0.16 | 0.570† |
| MIP_T0 | 112.96 ±16.62 | 110.24 ±18.75 | 0.590† |
| MEP_T0 | 136.00 ±25.39 | 144.86 ±23.81 | 0.218† |
Pulmonary function
Repeated measures ANOVA were used to test treatment and time effects on the mean spirometric values within groups. The mean spirometric values of FEV1, FVC, and FEV1/FVC increased after 3 weeks of treatment with chest physical therapy for patients in group A at T1 compared with their baseline mean values at T0 (p < 0.05).
Follow-up of patients in group A at 3 weeks after HSCT (T2) revealed that their mean values of FEV1, FVC, and FEV1/FVC had decreased slightly compared with T1 (p > 0.05) but was still significantly increased compared with the baseline (T0) measures (p < 0.05). Regarding the control group, the mean values of FEV1, FVC, and FEV1/FVC had decreased markedly at both T1 and T2 compared with the baseline T0. On comparing the two groups at T1 and T2, the mean spirometric values of FEV1, FVC, and FEV1/FVC were improved significantly in group A more than in group B (p < 0.05, Table II).
Table II
Spirometric values (FEV1, FVC, FEV1/FVC) in both groups
| Time of evaluation | Group A | Group B | P-value |
|---|---|---|---|
| FEV1: | |||
| T0 | 2.93 ± 0.55 | 2.97 ±0.41 | 0.773† |
| T1 | 3.40 ±0.52 | 2.86 ±0.47 | 0.000† |
| T | 3.34 ±0.60 | 2.80 ±0.44 | 0.001† |
| P-value | < 0.001* | 0.046* | |
| FVC: | |||
| T0 | 4.17 ±0.46 | 4.12 ±0.53 | 0.735† |
| T1 | 4.42 ±0.42 | 4.02 ±0.50 | 0.004† |
| T2 | 4.29 ±0.44 | 3.86 ±0.61 | 0.007† |
| P-value | 0.017* | 0.062* | |
| FEV1/FVC: | |||
| T0 | 0.71 ±0.15 | 0.73 ±0.16 | 0.570† |
| T1 | 0.77 ±0.13 | 0.73 ±0.18 | 0.268† |
| T2 | 0.79 ±0.16 | 0.75 ±0.18 | 0.377† |
| P-value | 0.045* | 0.758* | |
Respiratory muscle strength measures (MIP, MEP)
Table III shows the mean values of maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP) before treatment at T0 and after treatment at T1. Comparison of these measures indicates that they increased significantly after 3 weeks of treatment (T1) with chest physical therapy for patients in group A compared with their baseline mean values (T0), p < 0.05. Follow-up of patients in group A at 3 weeks after HSCT at T2 revealed that the mean values of MIP and MEP had decreased slightly compared with T1 (p > 0.05) but were still significantly higher than the baseline (T0; p < 0.05). For the control group B, the mean values of MIP and MEP had decreased markedly at both T1 and T2 compared with the baseline (T0). Comparing both groups at T1 and T2, the mean values of MIP and MEP had both improved significantly more in group A than in group B (p < 0.05).
Table III
Respiratory muscle strength (MIP, MEP) in both groups
| Time of evaluation | Group A | Group B | P-value |
|---|---|---|---|
| MIP: | |||
| T0 | 112.96 ±16.62 | 110.24 ±18.75 | 0.590† |
| T1 | 135.84 ±13.43 | 105.72 ±16.58 | 0.000† |
| T2 | 132.20 ±16.07 | 103.60 ±15.35 | < 0.001† |
| P-value | < 0.001* | 0.082* | |
| MEP: | |||
| T0 | 136.00 ±25.39 | 144.86 ±23.81 | 0.218† |
| T1 | 152.44 ±19.14 | 134.84 ±24.33 | 0.007† |
| T2 | 147.92 ±19.29 | 129.76 ±23.02 | 0.003† |
| P-value | 0.001* | < 0.001* | |
Discussion
Allo-HSCT survivors are unique patients who need special care due to the effects of chemotherapy and radiotherapy received before the transplant, which often result in various complex medical problems such as respiratory muscle weakness and functional capacity reduction. Weakness of expiratory muscles may cause inefficient gas exchange, powerless cough, and the accumulation of secretions, all of which may contribute to postoperative pulmonary complications such as atelectasis and pneumonia [34]. Chest physical therapy can be helpful not only in prevention but also in the treatment of respiratory dysfunctions in these unique patients [35–38].
Performing chest physical therapy before transplantation should be an essential part of the pre-transplant rehabilitation process. There is a gap in the literature regarding the precise role of CPT pre-HSCT and its impact on PF and RMS before and after HSCT. Therefore, the present study was carried out to investigate the safety, feasibility, and effects of pre-transplant CPT on PF and RMS before and after HSCT.
The findings of the present study revealed that the mean spirometric values of FEV1, FVC, and FEV1/FVC, as well as MIP and MEP, all improved significantly more in the CPT group than in the control group after 3 weeks of treatment at T1, immediately before HSCT.
Follow-up of the intervention group at 3 weeks after HSCT (T2) revealed that the mean values of FEV1, FVC, and FEV1/FVC, as well as MIP and MEP, had decreased a little compared with T1 but were still significantly increased in comparison with the baseline measures at T0, and compared with the control group (p < 0.05). These results suggest that a CPT protocol applied before the transplant can improve PF and RMS before HSCT, as well as preventing a significant decrease of them after HSCT. Improvements in PF in the CPT group might be attributed to the CPT program, which was used to improve their ventilation efficiency and prevent lung infection by improving alveolar ventilation, venous return, and lymph waste, and decreasing dead space ventilation [39].
Suesada et al. (2007) found a significant reduction in MIP in patients who did not undergo respiratory exercises within 5 days of hospitalization [40]. Furthermore, other previous studies have also observed ventilation-related diaphragmatic dysfunction and a decrease in contractile fiber units due to disuse after only 12 h of mechanical ventilation [41–43].
Using incentive spirometry as a component of the CPT program might increase RMS and endurance. The increase in RMS may be due to increased recruitment of motor units. IS also improves the efficiency of ventilation, gives visual feedback for diaphragmatic training, maintains positive pressure in the airways, and increases perfused alveoli. While performing breathing exercises with IS, the patient should mobilize a large tidal volume with a low respiratory rate to improve muscle strength via an increased inspiration/expiration ratio. The maximum inspiration the patient took while using IS may increase the transpulmonary pressure and pulmonary volume, additionally, the rest at the end of inspiration keeping up this increase and ensuring alveolar stability [44, 45]. Exercises using IS have many benefits; they are simple, cheap, safe, and have no known side effects. Another benefit is that once the patient is educated on how to use the method, no further supervision is needed [46].
Some studies have found that IS improves pulmonary functions (VC, FEV1, PEFR, and MVV), as it reduces the resistance to airflow by increasing lung volume, improving deep diaphragmatic breathing, and improving the expansion of collapsed areas [47]. Feedback respiratory training can improve respiratory function, exercise capacity, dyspnea perception and quality of life, and is especially motivating for children [48].
Bom et al. administered a pulmonary rehabilitation protocol that included inspiratory muscle training immediately after HSCT and continuing over a seven-day treatment period, and reported improvement in RMS [49]. In the current study, PF and RMS improved significantly after a CPT program that was applied for 3 weeks before the transplant, with large treatment effects seen after HSCT.
There are some limitations to the current study including sample size, local site recruitment, and the absence of a control group that did not receive any intervention; also, the type of conditioning regimen was not considered, the time that the participants spent out of bed and the hospitalization duration were not assessed, and follow-up assessment after discharge was lacking. The study did not include measurements of immune outcome. Another limitation was that the physiotherapist was not blinded to the study groups.
In conclusion, the current study highlights the positive impact of a 3-week CPT protocol applied before transplantation in improving pre-transplant pulmonary function and respiratory muscle strength and preventing their post-transplant reduction in allo-HSCT recipients. CPT is recommended to be applied during the pre-transplant waiting period, not merely to prevent the decline in pulmonary function and respiratory muscle force but also to increase gains in these measures.