Introduction
Glutathione reductase (GR) is a homodimeric flavoprotein composed of 52 kDa monomers [1, 2]. In addition to its synthesis, the activity of GR provides a secondary source of reduced glutathione (GSH) in the cytosol and certain organelles, such as the mitochondria [1, 2]. GR catalyzes the conversion of oxidized glutathione (GSSG) to GSH, maintaining high levels of GSH and low levels of GSSG within the organism, thereby preserving an appropriate reducing environment [1, 2]. Recent studies have highlighted the close relationship between GR levels in cells or blood and diseases such as Alzheimer’s disease, Parkinson’s disease, liver cancer, and colon cancer [3–6]. In particular, serum GR activity is significantly elevated in patients with infectious hepatitis, acute hepatitis, cirrhosis, and liver metastasis [7, 8]. Therefore, monitoring serum GR activity holds potential clinical value for the auxiliary diagnosis and prognosis of these conditions [3–8]. Thus, measuring serum GR in the laboratory can provide crucial support for the diagnosis and management of related diseases.
The reference interval is a critical component of laboratory reports, providing a fundamental basis for evaluating whether laboratory indicators fall within normal ranges. It plays a pivotal role in clinical decision-making, particularly in disease diagnosis and treatment [9, 10]. Currently, reference intervals for serum GR are predominantly derived from manufacturer guidelines. However, these intervals often lack broad applicability due to small sample sizes and insufficient stratification by gender and/or age. Consequently, there is a pressing need for laboratories to establish reference intervals that account for the specific lifestyle, gender, age, and methodological characteristics of the local population. Such tailored intervals would significantly enhance the clinical utility of laboratory indicators [11, 12].
To date, few studies have focused on the establishment of GR reference intervals by laboratories, and the sample sizes in these studies have generally been small [13, 14]. In this study, we utilized the ultraviolet enzymatic method to measure serum GR activity in apparently healthy adults aged 20 to 79 years. The distribution of GR activity was analyzed, and reference intervals for serum GR were established in a single-center population in Jiangsu, Eastern China, in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) C28-A3 and WS/T 402-2024 [15, 16]. This study aims to provide a valuable foundation for the auxiliary diagnosis and treatment of clinically related diseases.
Material and methods
Study subjects
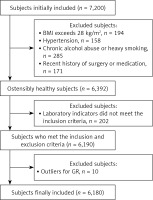
This study employed a completely randomized approach, initially selecting 7,200 individuals who underwent physical examinations at the Physical Examination Center of the Second People’s Hospital of Lianyungang between January 2024 and June 2024. Based on predefined inclusion and exclusion criteria, 6,180 apparently healthy individuals were ultimately included, comprising 2,996 males and 3,184 females, aged 20 to 79 years. The screening process for research subjects is illustrated in Figure 1.
The inclusion criteria for this study were as follows: (1) aged 20–79 years with a body mass index (BMI) between 18.5 and 28 kg/m2 (BMI ≥ 28 kg/m2 was classified as obese); (2) normal blood pressure, defined as systolic blood pressure < 140 mm Hg and diastolic blood pressure < 90 mm Hg; (3) clinical biochemical indicators within normal reference ranges, including albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), urea, creatinine (Cr), uric acid (UA), glucose (GLU), total cholesterol (TC), and triglycerides (TG); (4) negative immune markers for hepatitis A, B, C, and E; (5) complete blood count results within normal reference ranges; (6) no significant abnormalities detected through physical examination, chest X-ray, electrocardiogram, or abdominal ultrasound.
The exclusion criteria for this study were as follows: (1) digestive system diseases, including hepatitis, cirrhosis, fatty liver, cholelithiasis, cholecystitis, or inflammatory bowel disease; (2) acute or chronic infections, such as upper respiratory tract infections, pneumonia, or tuberculosis; (3) kidney diseases, including acute kidney injury and chronic kidney disease; (4) metabolic or nutritional disorders, such as diabetes, metabolic syndrome, hyperuricemia, or gout; (5) rheumatic diseases, including systemic lupus erythematosus and rheumatoid arthritis; (6) thyroid disorders, such as hyperthyroidism or hypothyroidism; (7) blood disorders, including leukemia and anemia; (8) recent history of blood donation, transfusion, significant blood loss, surgery, or medication use; (9) conditions such as malnutrition, vegetarianism, chronic alcohol consumption, heavy drinking within the past 2 weeks, or heavy smoking (≥ 20 cigarettes/day); (10) recent strenuous exercise or heavy physical labor; (11) pregnancy or breastfeeding in women.
Study design
Following the recommended methods outlined in the C28-A3 and WS/T 402-2024 guidelines [15, 16], subjects were initially categorized by gender. If a significant difference (p < 0.05) in serum GR activity was observed between genders, further subgrouping by age was performed. Both males and females were categorized into age groups: 20–29, 30–39, 40–49, 50–59, 60–69, and 70–79. If significant differences (p < 0.05) in serum GR activity were found among age groups, the reference interval was established based on age. If no significant differences were observed, the reference interval was determined by combining all age groups.
Specimen collection
All subjects adhered to the standard procedures outlined in the Guidelines for Venous Blood Specimen Collection [17], using the same batch of sampling needles and blood collection tubes. The procedure was as follows: subjects maintained a normal diet and regular routines for 3 days prior to blood collection. On the morning of the fourth day, 5 ml of fasting venous blood was collected using yellow-capped tubes with separation gel. The samples were left to stand for 30 min to allow coagulation, then centrifuged (relative centrifugal force: 1000–1200 g, duration: 10 min). Serum was separated within 2 h and used for biochemical assays.
Analyzers and reagents
In this study, serum GR activity and other biochemical analytes were measured using the AU5800 automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA) with the following commercial reagent kits: GR (lot: 20231027), ALB (lot: AUZ1574), ALT (lot: AUZ1963), AST (lot: AUZ2080), GGT (lot: AUZ2621), Urea (lot: AUZ2407), Cr (lot: 2541), UA (lot: AUZ2547), GLU (lot: AUZ2287), TC (lot: AUZ2280), and TG (lot: AUZ2697).
The detection methods used were: ultraviolet enzyme method for GR, bromocresol green method for ALB, rate method for ALT, AST, and GGT, urease-glutamate dehydrogenase method for urea, enzymatic method for Cr, uricase-peroxidase method for UA, hexokinase method for GLU, enzymatic method for TC, and phosphoglycerate oxidase-peroxidase method for TG. The GR test kit was manufactured by Jiangsu Maiyuan Biotechnology Co., Ltd., with a specified reference range of 33–73 U/l. The analyzer’s performance was verified, and both internal quality control and external quality assessments were conducted in accordance with required standards.
Principle of serum GR activity determination
Serum GR activity was determined using the ultraviolet enzyme method as specified by the kit manufacturer. The principle is as follows: GR catalyzes the reduction of GSSG to GSH, while reduced coenzyme II (NADPH) is oxidized to NADP+. NADPH has a specific absorption peak at a wavelength of 340 nm, and its oxidation rate is proportional to the GR activity in serum. The GR activity is calculated by measuring the rate of decrease in NADPH absorbance at 340 nm.
Methods for validation of reference intervals
In this study, the direct method was used to establish the reference interval for serum GR. Accordingly, a small sample size was utilized for verification, following the guidelines outlined in WS/T 402-2024 [16]. The verification process is summarized as follows: (1) At least 20 qualified reference individuals were selected for each group, ensuring a balanced distribution of gender and age. The screening criteria were consistent with those used during the establishment of the reference interval. (2) Specimens were collected, processed, and tested in accordance with the laboratory’s standard operating procedures. Prior to testing, the performance of the analytical system was verified to meet relevant requirements. (3) The Dixon method was applied to identify and exclude outliers. If the sample size became insufficient after outlier exclusion, additional samples were collected to maintain an adequate sample size. (4) Verification was considered successful if no more than 10% of the test results fell outside the established reference interval. If more than 10% of the data lay outside the interval, at least 20 additional qualified reference individuals were screened, and the verification process was repeated. (5) If verification was successful, the reference interval could be adopted. If verification failed, the laboratory re-evaluated the reference individuals and ensured that the analytical quality met the required standards.
Statistical analyses
Inspection of outliers
Following our previous report [18], the Dixon method was used to analyze and remove outliers.
Data analysis
Data analysis was performed using SPSS Statistics (IBM, Armonk, NY, USA), version 19. The Kolmogorov-Smirnov test was applied to assess the normality of the data. Data that conformed to a normal distribution were presented as mean ± standard deviation, while non-normally distributed data were reported as median (M) and interquartile range (IQR). For non-normally distributed data, comparisons between two groups were made using the Mann-Whitney U test, and comparisons among multiple groups were made using the Kruskal-Wallis test, followed by Dunn’s post hoc test. The reference interval for serum GR was determined using the two-sided percentile method (2.5th percentile to 97.5th percentile). A p-value of less than 0.05 was considered statistically significant.
Results
General information and parameters of reference individuals
According to the study’s inclusion and exclusion criteria, a total of 6,180 reference individuals were included. General information and indicators for all subjects, including gender, age, body mass index, blood pressure, as well as levels of ALB, ALT, AST, GGT, urea, creatinine, uric acid, glucose, total cholesterol, and triglycerides, are presented in Table I.
Table I
General characteristics of reference individuals
Distribution of serum GR based on gender and age
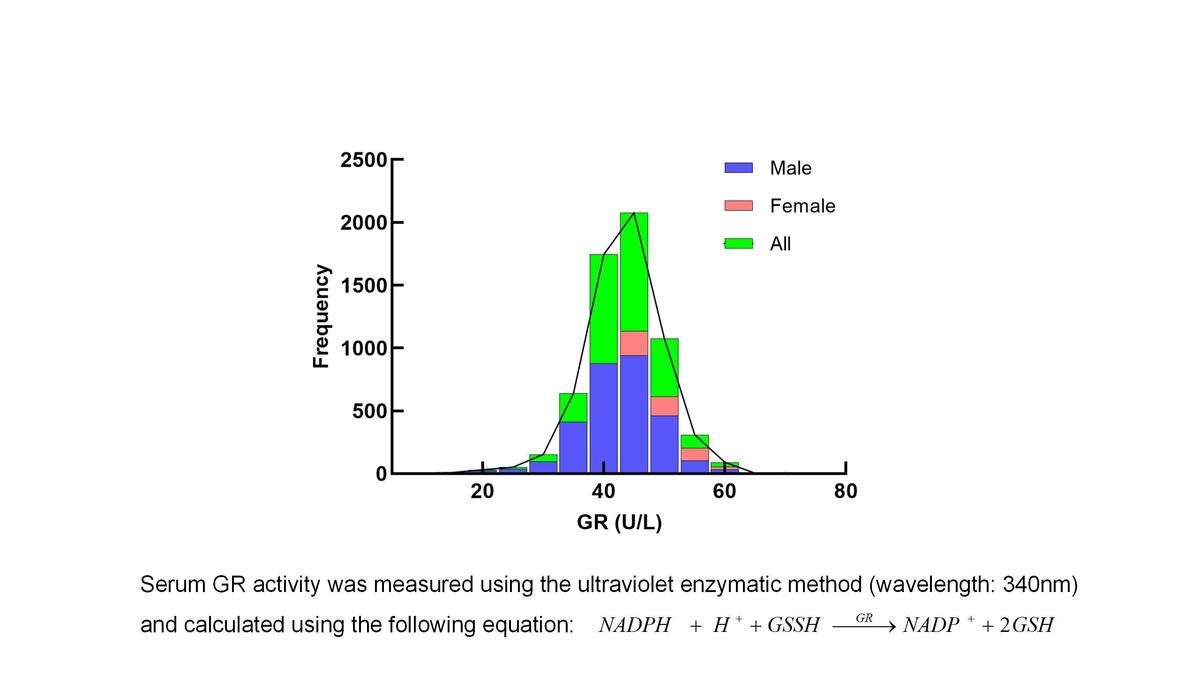
The Kolmogorov-Smirnov test indicated that serum GR activity in healthy adults was not normally distributed (Figure 2).
Initially, we compared serum GR activity between males and females. The analysis revealed a statistically significant difference in serum GR activity between genders (p < 0.05; Table II).
Table II
Distribution of serum GR by gender
Subsequently, the subjects were divided into six age groups to compare the distribution of serum GR activity and determine any age-related differences. The results showed no statistically significant differences in serum GR activity by age, for both males and females (all p > 0.05; Table III).
Table III
Distribution of serum GR by age
Establishment of reference intervals for serum GR
Based on the results, it was determined that gender factors should be considered when establishing the reference interval for serum GR, while age factors did not need to be considered. This study used a nonparametric method (two-sided percentile: 2.5th percentile to 97.5th percentile) to determine the reference interval for serum GR, in accordance with the C28-A3 and WS/T 402-2024 guidelines [15, 16]. Consequently, reference intervals for serum GR were established for apparently healthy adults in Eastern China using the ultraviolet enzyme method: 26.6–51.8 U/l for males and 29.7–55.3 U/l for females.
Additionally, to validate these reference intervals, we randomly selected samples from 20 males and 20 females, aged 20–79 years, all of whom were apparently healthy. For both males and females, over 90% of test results fell within the established reference intervals, demonstrating that the intervals determined in this study are appropriate for clinical use (Table IV).
Table IV
Validation results for serum GR reference interval
Discussion
Recently, the National Health Commission of China has established reference intervals for various laboratory indicators through multicenter studies, offering valuable insights into their clinical applications [19, 20]. It is important to recognize that the establishment of reference intervals is influenced by several factors, including population characteristics, geographical variations, and testing methodologies [21, 22]. Laboratories are required to verify the applicability of externally sourced reference intervals before adopting them [23, 24]. If verification is unsuccessful, it is recommended that laboratories establish reference intervals tailored to their specific regional populations [23, 24]. In this context, the present study is the first to investigate the distribution of serum GR activity in a single-center population of apparently healthy adults from Jiangsu province in Eastern China, and to establish its reference interval.
Based on the inclusion and exclusion criteria, a total of 6,180 reference individuals were included in this study. Our findings revealed that serum GR activity in healthy adults exhibited a non-normal distribution and significant gender differences, with females showing higher serum GR activity compared to males. After accounting for gender differences, the subjects were further stratified into age groups. The analysis indicated no statistically significant age-related differences in serum GR activity among healthy adults. In contrast, a study by Li et al. [13] reported slightly higher serum GR activity in men compared to women, though the difference was not statistically significant. Additionally, no significant age-related differences in serum GR activity were observed among men, whereas significant differences were noted among women. Conversely, Habif et al. [14], who investigated GR activity in erythrocytes from 130 healthy individuals, found no statistically significant differences in GR activity with respect to gender or age. The similarities and discrepancies between these studies and ours can be attributed to the following factors: (1) Population differences: The populations in the referenced studies were primarily from the Mediterranean region of Turkey and southern China, while the participants in this study were from eastern China. (2) Methodological variations: The referenced studies employed the GSSG substrate method or a modification of Beutler’s method, while our study utilized the ultraviolet enzymatic method. (3) Specimen types: Habif et al. [14] measured GR activity in erythrocytes, whereas our study assessed GR activity in serum. (4) Sample size differences: The referenced studies included 430 and 130 subjects, respectively, compared to the 6,180 individuals analyzed in this study [13, 14].
Based on the findings of this study and in accordance with the recommended methods outlined in the C28-A3 and WS/T 402-2024 guidelines, we established gender-specific reference intervals for serum GR activity in apparently healthy adults in eastern China: 26.6–51.8 U/l for males and 29.7–55.3 U/l for females. These intervals were validated using a smaller sample size, meeting the requirements for clinical application. Thus, the reference intervals established in this study provide valuable guidance for clinical practice. Comparatively, a study by Li et al. [13] preliminarily established reference intervals for serum GR activity in apparently healthy adults in Zhongshan, China, reporting ranges of 36–72 U/l for males, 38–65 U/l for females aged 18–50 years, and 45–67 U/l for females aged 51 years and older. Another study by Zheng et al. [25] cited the manufacturer’s upper limit for serum GR activity as 73 U/l. Furthermore, the GR reference interval provided by Jiangsu Maiyuan Biotechnology Co., Ltd., the manufacturer of the GR kit used in this study, is 33–73 U/l. These intervals are generally higher than those established in our study. Both previous studies and our research underscore that serum GR reference intervals are closely influenced by factors such as regional characteristics, population demographics, sample size, and methodological variations. Therefore, laboratories must consider these factors when establishing reference intervals to maximize the clinical utility of laboratory indicators.
While this study provides significant insights, it has several limitations: (1) Restricted age range: The study included only healthy adults aged 20 to 79 years, excluding specific groups such as pregnant women, infants, and adolescents. Consequently, the established reference intervals may not apply to these populations. (2) Single-center design: Conducted at a single center with participants predominantly from the eastern coastal regions of China, the results may have limited applicability beyond this geographic area. Other laboratories should validate these reference intervals before application. (3) Narrow focus: The study concentrated exclusively on serum GR activity in apparently healthy individuals and did not investigate its role in diagnosing or managing clinically related diseases such as hepatitis, cirrhosis, or liver cancer. (4) Methodological limitations: This study employed an ultraviolet enzymatic method to assess serum GR activity. However, this approach does not measure GR activity directly but rather infers it indirectly based on the rate at which oxidized glutathione is reduced to its reduced form. To further enhance the clinical utility of serum GR, future studies should include a broader range of populations, such as children, adolescents, and pregnant women, as well as participants from diverse geographic regions. Additionally, research should explore the role of serum GR in the diagnosis and management of related diseases to provide a more comprehensive understanding of its clinical relevance.
In conclusion, based on a single-center population in the Jiangsu region of eastern China, we established gender-specific reference intervals for serum GR in apparently healthy adults for the first time. These reference intervals will serve as a crucial guide for health screening and support the diagnosis and treatment of clinically relevant diseases.