Introduction
The adipose tissue is considered the largest endocrine “organ” of the human body. The higher the body mass is, the greater is the risk of type 2 diabetes mellitus and cardiovascular diseases, and also certain neoplasms.
Over 50 hormones and cytokines are synthesized and secreted by the adipose tissue [1]. In numerous reports data concerning a relationship between obesity and many cancers have been published.
In the obese carcinogenesis is mediated through insulin resistance, hyperinsulinemia, and increased levels of insulin-like growth factor 1 (IGF-1) [1, 2]. In vitro studies unambiguously indicate a proliferative effect of insulin, which also inhibits apoptosis and stimulates the synthesis of IGF-1, a pro-neoplastic hormone [2].
Relationships between adipokines and carcinogenesis are also sought in neoplasms not associated with obesity [2, 3]. Therefore, the authors analyzed adipokine receptors in the human adrenal tumors, expression of which has not been widely investigated so far.
Although adiponectin is a key mediator in the development and progression of various cancers, the underlying pathomechanisms connected with neoplasia are still poorly understood [1, 2]. Research on relationships between various adipokines and endocrine neoplasms is ongoing.
Expression of adiponectin (AdipoR1 and AdipoR2) and leptin (Ob-R) receptors in adrenal tumors has not been widely investigated so far.
The authors of the current study were the first to retrospectively analyze the expression of adiponectin (AdipoR1 and AdipoR2) as well as leptin (Ob-R) receptors in adrenal tumor samples of patients who had been operated on in a single clinical center.
Material and methods
The study included 128 patients (87 females, 41 males) diagnosed with adrenal tumors who had been hospitalized in the Department of Endocrinology and Internal Medicine of the Medical University of Gdansk between 1995 and 2014. Patients were aged 23 to 92 (mean age: 52.5 years; females 53 years; males 51.3 years).
In the group of operated tumors, adrenal adenomas (CA) were diagnosed in 28 cases, cortical hyperplasia tumors (CNH) in 35 cases, pheochromocytomas (PHEO) in 20 cases and malignant pheochromocytomas (PHEOM) in 5 cases.
All groups of patients were found to have comparable body mass index (BMI) (CC – BMI median: 26 kg/m2; CNH/CA – BMI median: 27 kg/m2; PHEO/PHEOM – BMI median: 26 kg/m2).
The study material comprised tissue specimens of resected adrenal tumors. The histological type of the tumor was determined according to the 2004 World Health Organization Classification (Pathology and Genetics of Tumours of Endocrine Organs, Lyon, 2004).
Tumor tissue was preserved according to standard procedures (fixed for approximately 48 h in 10% formaldehyde solution, later dehydrated using appropriate alcohol and xylene solutions, and, finally, embedded in low melting point paraffin). Tissue specimens of analyzed cases were re-assessed in routinely prepared hematoxylin-eosin (H + E) stains to verify the histopathological diagnosis. From the studied specimens fragments most representative for neoplasm were chosen; there were neither necrotic lesions nor thermal damage areas in these fragments.
The selected specimens along with respective paraffin blocks were used to map tumor areas extracted for tissue microarrays. This was done using 1.5-mm diameter needles. Tumor cores were inserted in pre-prepared “recipient” paraffin blocks that contained no tissue. The complete tissue microarrays were created using Manual tissue arrayer I by Beecher Instruments (MTAI, K7 BioSystems). At least three cores (biopsies) of neoplastic tissue were removed. This way at least ten microarrays were acquired. Additionally, in every newly created recipient block, cores of palatine tonsil and stomach wall were inserted, which served as an internal control and facilitated orientation among cores acquired from different specimens within one block. This orientation method allowed for localizing a specific core of a given tumor specimen.
The study was confirmed by the Independent Ethic Committee of the Medical University of Gdansk (NKBBN/360-98/2016).
Preparation of specimens
Immunohistochemical stains were made with microtome sections of 4 micrometer thickness. They were placed on Superfrost PLUS slides (by Surgipath) and later incubated overnight at 37ºC.
Antibodies used for detection along with applied methodology are presented in Table I.
Table I
Antibodies used for detection along with applied methodology
Assessment of immunohistochemical reactivity and statistical analysis
Antigen expression was assessed in three samples acquired from the same tumor. The assessment comprised the percentage of cells with positive reactivity (0–100%) as well as the intensity of reactivity (0–3), and was expressed as their product (H-score). For each tumor three scores were calculated, and the highest of these was considered final.
H-score values were classified as follows: 0 to 100 denoted lack of or low antigen expression, 101 to 200 denoted moderate antigen expression, and 201 to 300 denoted high antigen expression.
Statistical analysis
Standard descriptive statistics were calculated. Differences between groups were determined using linear regression with robust standard errors. Correlations were assessed using Spearman’s method. P-values lower than 0.05 were considered statistically significant. Calculations were performed with STATA 13.1 software by StataCorp (USA, Texas).
To increase the reliability of the test results, two independent pathologists evaluated the results of immunohistochemistry in tissues of adrenal tumors. All ACC cases are defined according to the Weiss criteria.
Results
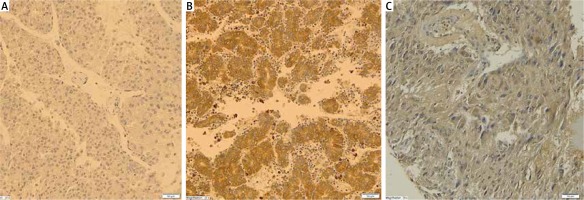
In two thirds of cortical adenoma and cortical nodular hyperplasia cases absence of or low AdipoR1 receptor expression was revealed. Its expression was significantly higher in cortical carcinomas (p < 0.001) and pheochromocytomas (p < 0.001). There was no statistically significant difference between AdipoR1 expression in carcinomas and pheochromocytomas (Table II, Figure 1).
Table II
Expression of adiponectin and leptin receptors in various adrenal tumors by immunohistochemistry
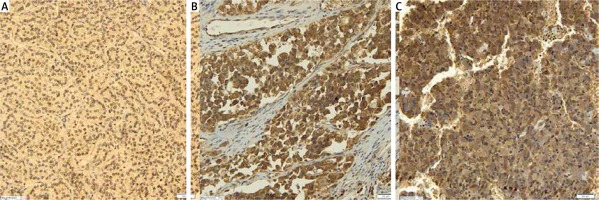
AdipoR2 was at least moderately expressed in all types of tumors. This receptor’s expression was significantly higher in cortical carcinomas than in adenomas combined with nodular hyperplasia tumors (p = 0.010), as well as significantly higher in pheochromocytomas compared to cortical carcinomas (p = 0.004) (Table II, Figure 2).
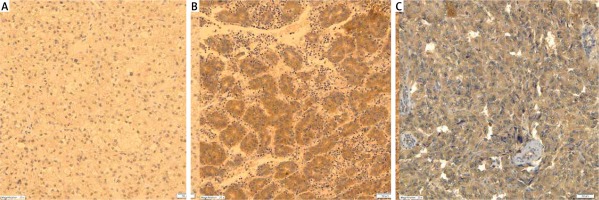
Leptin receptor expression was absent or low in half of nodular hyperplasia tumors and adrenal cortex adenomas. Its expression was significantly higher in cortical cancers (p = 0.038). In pheochromocytomas this receptor was expressed more abundantly than in adrenocortical carcinomas (p = 0.004) (Table II, Figure 3).
Using retrospective data, the authors assessed the relationship between the expression of adrenal adipokine receptors and patient’s sex and BMI.
There was no statistically significant difference between adrenal adipokine receptor expression levels and gender of the patients (AdipoR1 p = 0.846; AdipoR2 p = 0.521; Ob-R p = 0.642). There was a significant difference between BMI and adipokine receptors’ expression. Although higher BMI index was associated with lower adipokine receptors’ expression (AdipoR1 p = 0.0337; AdipoR2 p = 0.0001; Ob-R p = 0.0008), the BMI index was similar in each histopathological group.
Discussion
The role of adipokines in the development of human endocrine neoplasms has not been elucidated. There have been few studies in which expression of adiponectin and leptin receptors in adrenal gland neoplasms in humans was investigated. However, the relationship between hormonal activity of adipose tissue and tumorigenesis as well as hormonal activity of adrenal tumors seems significant [1, 2].
The authors of one of the most recent studies showed that the adipose tissue surrounding adrenal glands actively secretes leptin and adiponectin [4]. Local adipokine secretion may have an effect on adrenal tumor pathophysiology [4].
ADIPOR1 and ADIPOR2 genes are expressed physiologically in human adrenal glands. Two recently discovered adiponectin receptors, AdipoR1 and AdipoR2, mediate the biological effects of the adipose tissue-derived protein adiponectin [5].
Adiponectin receptors were expressed in many obesity-associated and -unassociated human neoplasms. Significantly higher AdipoR1 and AdipoR2 expression was found in patients with obesity-associated neoplasms compared to those neoplasms that probably are not etiologically associated with obesity, such as cervical squamous cell carcinoma, adrenocortical carcinoma [2] or thyroid cancer [3].
In our study, differential AdipoR1 and AdipoR2 expression in histopathologically distinct adrenal tumors from patients operated on in a single clinical center was examined for the first time. We found significantly higher AdipoR1 and AdipoR2 expression in adrenocortical cancer compared to benign neoplastic growth, i.e. cortical nodular hyperplasia and adenoma.
Several possible mechanisms may account for the role of adipokines in neoplasia. Elevated adiponectin plasma concentration improves insulin sensitivity in the obese. Therefore, in the case of low adiponectin levels elevated serum insulin and IGF 1 levels lead to increased proliferation, which also applies to neoplastic cells [1, 2, 6]. Adiponectin selectively binds with multiple mitogenic growth factors, which ultimately affects the regulation of proliferation, differentiation, and apoptosis through 5′-AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR), and c-Jun NH2- terminal kinase and STAT3 (JNK-STAT3) pathways [7–9]. Adiponectin is also an angiogenesis inhibitor in endothelial cells [2, 7]. In recent studies the role of AdipoR1 and AdipoR2 in neoplasms has been investigated. Ex vivo research showed that by binding with AdipoR1 adiponectin may attenuate the pro-neoplastic effect of leptin by its inhibitory action on the leptin receptor in the esophageal neoplasm [8, 10], or by inhibiting the progression to the S (synthesis) phase in breast cancer [11]. There are, however, no studies explaining the role of AdipoR2 in human neoplasms.
Increased adiponectin receptor expression in malignant adrenal tumor tissue may be explained by the down-regulation mechanism, which is present and has been elucidated in breast epithelial cancer [12] and Barrett’s adenocarcinoma [13]. In the case of the current study, the authors deduced that low plasma adiponectin levels, associated with pro-neoplastic effects, lead to increased AdipoR1 and AdipoR2 expression in adrenal tumor tissue, with higher expression found in cortical cancers.
Isobe et al. were the first to reveal the presence of AdipoR1 and AdipoR2 in human pheochromocytoma (PHEO) tissue [14]. Pheochromocytoma are tumors that produce catecholamines, which results in reduced glucose tolerance. Diabetes is present in one third of patients with PHEO. The relationship between diabetes and pheochromocytoma encompasses suppression of insulin secretion by catecholamines and induction of insulin resistance [14].
So far, no studies have compared adiponectin receptors’ expression in adrenal cortex and medulla neoplasms. We showed that AdipoR1 and AdipoR2 expression was significantly higher in pheochromocytomas compared to adrenocortical tumors. We found no statistically significant differences between the expression of adiponectin receptors in benign and malignant PHEO. It should be remarked that there were only five cases of malignant pheochromocytomas in the studied group.
Adrenaline has an up-regulatory effect on adiponectin receptor expression. It seems that high catecholamine concentrations found in PHEO, which cause glucose intolerance and diabetes in 1/3 cases, induce an intense compensatory increase in the expression of adiponectin receptors, AdipoR1 in particular, in PHEO tumor tissue, most likely due to a decrease in adiponectin plasma concentration in pheochromocytoma patients [14]. The authors of the current study speculate that catecholamine-mediated adiponectin suppression is more intense than the supposed effect of hormones secreted by adrenocortical tumors.
Leptin is one of the crucial factors that govern energy balance and body weight in humans. It is mainly synthesized by differentiated adipocytes, and it stimulates intracellular lipolysis of triglycerides of muscle cells, while it inhibits lipolysis in the liver and pancreatic islets [15]. Acting on its receptors located in the β-cells of pancreatic islets, and inhibiting the activation of ATP-dependent potassium channels, leptin reduces insulin synthesis. This hormone also has a role in the development of insulin resistance [15]. Expression of the leptin receptor Ob-R has been found in the adrenal glands of rats, mice and humans [16, 17].
Prior to the current study, a comparison of leptin receptor expression in histopathologically distinct adrenal cortex and medulla tumors has not been carried out. The authors of this report found no statistically significant differences in leptin receptor expression between benign and malignant pheochromocytomas, whereas leptin receptor expression was significantly higher in adrenocortical cancers compared to benign cortical neoplasms, i.e. nodular hyperplasia and adenoma of the adrenal cortex.
Mechanisms underlying the oncogenic effect of leptin have not been clarified thus far. It has been shown that the hormone stimulates the growth of neoplastic cells in esophageal, stomach, pancreatic, prostate, ovarian, and lung cancers [15]. The authors of another report suggested a relationship between leptin level and stimulation of proliferation of colon cancer cells [18]. It has also been indicated that leptin promotes proliferation of certain (not all) breast neoplasms in vitro, and promotes tumor invasiveness and angiogenesis in some animal models [18, 19]. It seems that pro-neoplastic effects of leptin are more strongly pronounced in neoplasms associated with obesity (esophageal, breast, stomach, colon, and pancreatic cancers) compared to those in which obesity plays a lesser role [8].
An attempt at an assessment of the proliferative effect of leptin in adrenal tumors was undertaken by Glasgow and coworkers as early as 1999 [17]. They examined normal and cancerous tissue of the adrenal cortex. These researchers found that although the leptin receptor is present in adrenal tumors, leptin does not regulate the proliferation of neoplasms [17].
In our study a comparison between leptin receptor expression in adrenal cortical and medullary neoplasms was made. We found that this receptor’s expression is significantly higher in PHEO than in adrenocortical tumors.
In a study by Bottner et al. it was shown that chronic supraphysiological synthesis of catecholamines present in PHEO patients does not lower the leptin level. It may be associated with tolerance of the adipose tissue to chronic catecholamine excess [20]. Mutual relationships between surrounding fat tissue and leptin secretion stimulation by catecholamines may be connected with increased expression of leptin Ob-R receptor in PHEO.
The retrospective results of our research should be correlated with plasma adiponectin and leptin levels in patients with malignant or benign adrenal tumors in future prospective studies.
In conclusion, the authors of this study were the first to compare expression of adiponectin and leptin receptors’ expression in histopathologically distinct adrenal tumors. Despite the fact that the expression of these receptors in adrenal tumors not associated with obesity is lower compared to neoplasms in which obesity is a vital etiological factor, the statistically significant differences in adiponectin receptors’ expression between cortical cancers and benign neoplasms suggest that adiponectin has a role in tumorigenesis also in these neoplasms. The authors also demonstrated that leptin receptor expression differs between malignant and benign adrenal neoplasms. Leptin receptor expression was, however, significantly higher in pheochromocytoma than in cortical carcinoma. It is possible that leptin’s impact on metabolic disorders is greater than on neoplasia. Higher adiponectin and leptin receptor expression in PHEO might result from a significantly greater effect of catecholamines on secretion of adipokines from adipose tissue than adrenal cortex hormones’ effect on this secretion.
The obtained results were independent of BMI and gender of the study groups.
The pathophysiological roles of adiponectin and leptin and their receptors, as well as the potential predictive value of these hormones in assessing the risk of malignancy, recurrence, and treatment results, require further prospective research.