Introduction
Spondyloarthritis (SpA) is a group of chronic inflammatory disorders that occurs mainly in HLA-B27-positive individuals, and is characterized by inflammatory back pain and entheseal involvement. In the past few years, the Assessment of Spondyloarthritis international Society (ASAS) has successively developed and validated the criteria for axial SpA (axSpA) and peripheral SpA [1, 2]. Ankylosing spondylitis (AS), the main subgroup of SpA, is characterized by specific radiographic changes in the sacroiliac joints. SpA occurs particularly in young men of working age and leads to a significant health burden to the community. If SpA is not diagnosed early and treated appropriately, more than 50% of the affected patients will develop AS within 5-10 years.
The treatment of SpA has advanced considerably with the emergence of tumor necrosis factor inhibitors (TNF blockers), which provide excellent symptomatic relief to many patients [3–5]. Although no data are available to date regarding the ability of biologics to prevent structural damage in axSpA, very early treatment appears to improve remission rates and retard structural progression [6, 7].
The etanercept biosimilar Yisaipu (YSP) is a recombinant human soluble TNF receptor and is one of the most widely used biosimilars in China. Several randomized clinical trials have shown that YSP is effective and safe for the treatment of AS [8, 9]. Like other biologics, YSP is an expensive therapy with a heavy economic burden for the health care systems. In addition, YSP may be associated with adverse events (AEs), such as tuberculosis and hepatitis B infections, which is of particular concern in China due to the high incidence of these two diseases. Therefore, we believe that any effective, ethical strategy to reduce the cost and risk of AS treatment but not worsening the quality of life of patients would be of great value. However, no guidelines are currently available on when to stop or how to taper the TNF blockers when the disease reaches remission [10–13]. With the availability of highly effective YSP, the question of possible reduction or discontinuation of TNF blocker therapy has taken on greater importance. In the present study, we investigated whether dose reduction or discontinuation of YSP may be possible after achieving remission in the early stage of axSpA in a real-world setting.
Material and methods
Patients and treatment regimen
From August 2015 to July 2016, 144 patients with early axSpA and symptom duration of ≤ 3 years were enrolled from the Rheumatology Department of our hospital. Patients were asked to participate if they had been treated with YSP 50 mg once weekly (QW) for at least 24 weeks and had achieved remission (Ankylosing Spondylitis Disease Activity Score (ASDAS-CRP) ≤ 1.3) for more than 12 weeks at baseline. Patients who satisfied the inclusion criteria entered one of the following three treatment arms: full dose (YSP50), half dose (YSP25), and withdrawal (YSP0) of YSP. Patients were matched 1 : 1 : 1 by propensity score matching of baseline data, including sex, age, symptom duration, HLA-B27, non-radiographic axSpA, smoking status, educational status, income, regular exercise, ASDAS-CRP prior to YSP initiation, ASDAS at baseline, duration of YSP treatment, C-reactive protein, and concomitant nonsteroidal anti-inflammatory drug (NSAID) and/or disease-modifying antirheumatic drug (DMARD) therapy. NSAIDs or DMARDs were maintained at the same dose throughout the study.
The study protocol was approved by the local ethics committee. Before enrollment, each patient was informed of the study nature, duration, and purpose, as well as all the potential benefits and risks. All participants provided written informed consent.
Definitions
Disease remission was defined as an ASDAS-CRP score ≤ 1.3 [14]. Disease relapse was defined as an ASDAS-CRP score > 1.3 at two consecutive visits or any new emergence of peripheral articular and extra-articular manifestations. Patients who requested to resume their initial regimen due to self-reported discomfort without the above parameters were also considered to have a disease relapse.
Assessments and endpoints
Patients were assessed by the same rheumatologist every 8 weeks for 48 weeks. If a flare occurred during the study, the patient resumed YSP 50 mg weekly or was switched to another TNF blocker. Patients who discontinued the study for any reason were designated as failures in the primary analysis. The date of the last visit was defined as the end of follow-up.
The primary endpoint was the proportion of non-failure patients in each group. The secondary endpoints included the time from baseline to failure, the proportion of patients regaining remission after a flare, cost of drugs, and the number and severity of AEs.
Adverse events
All patients were monitored for clinical and laboratory evidence of AEs throughout the study.
Statistical analysis
Descriptive analyses were performed for the demographic and clinical variables. The results are reported as mean ± SD for continuous, normally distributed data, as median (P25, P75) for non-normally distributed data, and as relative frequencies for categorical variables. Continuous data were compared among groups by analysis of variance. Frequency data were compared using the χ2 test. P-values less than 0.05 were accepted as statistically significant. All statistical analyses were performed using the SPSS 21.0 software.
Results
Patient characteristics
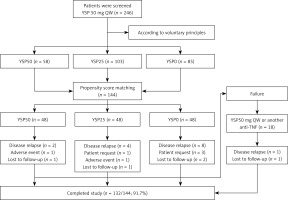
A total of 246 patients were screened and were divided into the YSP50 group (n = 58), YSP25 group (n = 103), and YSP0 group (n = 85) according to the voluntary principle. Of the 246 patients screened, 144 patients entered the study by propensity score matching (Figure 1). No significant differences in the demographic or clinical characteristics were observed between the three groups. Table I summarizes the patient demographic and clinical characteristics at baseline.
Table I
Demographic and clinical characteristics of the 144 patients at baseline
Clinical response
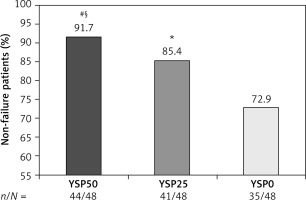
The proportion of non-failure patients was 91.7% (44/48), 85.4% (41/48), and 72.9% (35/48) in the YSP50, YSP25, and YSP0 groups, respectively, and the difference in overall non-recurrence rate was statistically significant across the three groups (p = 0.043; Figure 2). However, the results of the multiple-comparison test were not consistent. The proportion of non-failure patients was significantly higher in the YSP50 group than in the YSP0 group at 48 weeks (91.7% vs. 72.9%, p = 0.032). The difference for the other two comparisons was not statistically significant (YSP50 vs. YSP25 group, p = 0.522; YSP25 vs. YSP0 group, p = 0.132). Furthermore, the median time to flare was slightly longer in the YSP50 group (28 weeks) than in the YSP25 group (24 weeks) and the YSP0 group (24 weeks), but the difference was not statistically significant (p = 0.242).
Figure 2
Proportion of non-failure patients at 48 weeks. Overall non-recurrence rate (p = 0.043). Multiple-comparison testing (#p = 0.032, YSP50 vs. YSP0; §p = 0.522, YSP50 vs. YSP25; *p = 0.132, YSP25 vs. YSP0). YSP, Yisaipu (etanercept biosimilar)
A total of 14 patients experienced disease relapse, with 2 patients, 2 patients, and 1 patient presenting with acute anterior uveitis, peripheral arthritis, and psoriasis, respectively. Additionally, 4 patients requested to resume their initial treatment dose due to self-reported discomfort without disease relapse. Sixteen of these 18 patients regained remission rapidly after resuming YSP 50 mg QW or starting adalimumab 40 mg every other week (Figure 1).
The average annual costs of therapy in the YSP0 group (¥ 5375 ±735) and the YSP25 group (¥ 44567 ±2437) were significantly lower than in the YSP50 group (¥ 84724 ±3246) (p < 0.001). The difference between any two of the three groups was significant (YSP50 vs. YSP25 group, p < 0.001; YSP50 vs. YSP0 group, p < 0.001; YSP25 vs. YSP0 group, p < 0.001).
Adverse events
Adverse events were consistent with the known side effects of YSP, DMARDs, and NSAIDs. During the study, 64 (44.4%) patients experienced 95 AEs, including injection site reactions, infections (including tuberculosis infection), gastrointestinal disorders, hepatobiliary disorders, and blood system disorders. Of these AEs, injection site reactions and infections occurred more frequently in the YSP50 and YSP25 groups. Injection site reactions were seen in 8 patients in the YSP50 group and 5 patients in the YSP25 group. Upper respiration infections occurred in 8 patients in the YSP50 group, 6 patients in the YSP25 group, and 1 patient in the YSP0 group. Two patients (one each in the YSP50 and YSP25 groups) withdrew from the study due to tuberculosis infections that were resolved after 9 months of anti-tuberculosis therapy. The three groups demonstrated similar rates of other AEs. Gastrointestinal disorders occurred in 8, 10, and 11 patients, along with elevated liver enzymes in 7, 6, and 9 patients, and blood system disorders in 5, 5, and 4 patients in the YSP50, YSP25, and YSP0 groups, respectively. Except for the 2 tuberculosis infections, AEs were mild to moderate, not requiring interruption of the YSP, DMARD, or NSAID therapy. No deaths occurred during the course of the study.
Discussion
With the widespread application of TNF blockers, remission has not been a major focus in the treatment of axSpA [3–5, 15]. Remission is not only an indication of successful management of the disease, but also a possible indication to withdraw or reduce the dosage [16]. In clinical care, most patients with active axSpA are treated with a combination of at least two medications including a TNF blocker. In general, discontinuation or reduction of the dose involves only one medication at a time. Usually, but not always, the biological agent is the first to be discontinued or reduced with the aim of minimizing the costs and the risk of potential AEs [10, 11].
In the early stage of TNF blocker administration, a series of studies investigated the optimal strategy for these drugs in the management of patients with axSpA (including AS). To date, there is only limited and inconsistent evidence for TNF blocker dose adjustment (including tapering and stopping TNF blockers) for patients with axSpA in remission. Most of these studies were about etanercept therapy due to its longer-term use [17–22].
Several large-scale clinical trials of etanercept have reported that the relapse rates after etanercept withdrawal were high and variable. Clinical relapse was found in 50% to 95% of patients with different symptom duration after 1 year without TNF blockers. No difference was found between patients with early axSpA and established AS [17–19]. Studies on tapering strategies are relatively common and have mainly focused on dose reduction and extension of the treatment interval. Several retrospective and prospective clinical studies found no significant increase in disease flares between patients treated with etanercept at a reduced dose and those with a longer interval, with 70% to 90% of patients maintaining remission over 6–12 months of follow-up [20–22].
To the best of our knowledge, our study is the first prospective three-arm observational trial comparing the relapse rates of early axSpA patients in remission on an etanercept biosimilar across three different treatment groups (full dose, half dose, and discontinuation) in a real-world clinical setting.
In our study, from the full dose group to the tapering and discontinuation group, the proportion of patients who maintained a response showed a decreasing trend (91.7%, 85.4%, and 72.9%). The overall relapse rates of the three groups were statistically different, and the proportion of relapsed patients was significantly lower in the YSP50 group than in the YSP0 group. Our results suggest that a full dose is superior to YSP discontinuation but not superior to a half dose in patients with early axSpA in remission, while the half dose has a lower cost. Among the secondary endpoints, the median time to flare was not significantly different between the three groups (p = 0.242). Moreover, infections were more likely to occur in groups treated with YSP, whether YSP50 or YSP25, while rates of other adverse reactions were similar between the three groups.
It has been suggested that patients with early disease, low initial disease activity, and long time in remission are more likely to withdraw anti-TNF therapy successfully [23]. In our study, the proportion of non-failure patients at 48 weeks in every group was higher than that reported in previous studies. The relatively high remission rates in our patients may be related to the following facts. First, our patients had a disease duration of less than 3 years, and about 80% of them had non-radiographic axSpA. Second, our study used the ASDAS score for the definition of disease remission and flare. In comparison with other remission criteria such as the ASAS criteria and the Bath Ankylosing Spondylitis Disease Activity Index, the ASDAS includes subjective and objective measures of disease activity and may more accurately reflect disease activity by avoiding possible psychological influences. Third, most of our patients were receiving concomitant therapies including DMARDs, NSAIDs, and regular exercise, which may reduce disease flares. Finally, a number of studies have shown that Chinese patients with AS had a better response to TNF blockers than those in Western countries [4, 8, 9]. Thus, the rates of successful tapering or discontinuation in our study may be specific to the Chinese population. Even so, further studies are needed to confirm the results.
Beyond the potential curative effect and reduced drug exposure risk, stopping or halving the YSP dose after achieving remission can also reduce the economic burden either at the individual, medical insurance, or national level.
Our study has some limitations. The main weakness of our study is the limited sample size and the associated impact on statistical power. Another weakness is the lack of follow-up X-ray or magnetic resonance imaging (MRI) images due to the slow radiographic progression in axSpA patients and the desire to avoid repeated X-ray exposure in a short period. Moreover, the optimal time interval for repeated MRI during remission in axSpA is unclear, and MRI is a relatively expensive and time-consuming examination.
In conclusion, continuation with YSP at a full dose was not superior to a half dose in patients with early axSpA in remission for more than 12 weeks. The sustained disease remission observed after halving the YSP dose suggests that this therapeutic strategy may be applicable in clinical practice, with advantages in terms of drug exposure risk, patient compliance, and cost savings. From a practical standpoint, we recommend that early axSpA patients who achieve remission for more than 3 months may consider halving the dose of YSP for a period of transition before stopping the YSP treatment.