A 24-year-old Chinese woman visited our clinic presenting with recurrent rash and alopecia for 3 years. At 21 years old, she was diagnosed with systemic lupus erythematosus (SLE) based on the presence of a butterfly rash, alopecia, low levels of complement 3 (0.68 g/l; reference range 0.9–1.8 g/l) and complement 4 (0.08 g/l; reference range 0.1–0.4 g/l), a high titre of anti-nuclear antibodies (ANA, 1 : 320, homogeneous pattern; reference range < 1 : 80), and positive anti-Sm antibodies. Rash and alopecia went into remission after treatment with low-dose methylprednisolone (4 mg/day) and hydroxychloroquine (200 mg twice per day). She was prescribed hydroxychloroquine (200 mg/day) for maintenance therapy. At 23 years old, her butterfly rash relapsed and non-scarring patch alopecia occurred. Her symptoms did not improve with methylprednisolone (16 mg/day) combined with mycophenolate mofetil (750 mg twice per day) [1, 2], and hydroxychloroquine (200 mg twice per day) for 2 months. Considering that the JAK1/2 inhibitor baricitinib was effective in treating SLE with rash and alopecia areata [3, 4], she was given baricitinib (2 mg/day) for 3 months, but the alopecia was unchanged.
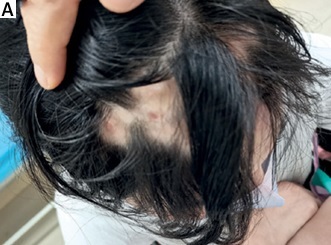
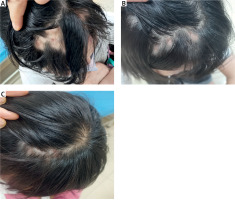
On physical examination, butterfly rash, chilblain-like rash on the ears and palms, and alopecia areata involving the front of the head were noted (Figure 1 A). Laboratory tests showed a white blood cell (WBC) count of 3.26 × 109/l (reference range 4–10 × 109/l), haemoglobin (Hb) of 106 g/l (reference range: 110–130 g/l), complement 3 of 0.76 g/l, and erythrocyte sedimentation rate (ESR) of 67 mm/h (normal range: 0–15 mm/h). Platelets (PLT), urinalysis, complement 4, Coombs test and 24-hour urine protein were all within normal ranges. Immunologic testing was positive for ANA (1 : 160, homogeneous pattern), anti-Sm, and anti-U1-RNP-70kd. She refused to undergo scalp skin biopsy. Due to the reported positive results of the telitacicept for SLE, telitacicept (160 mg per week, subcutaneous injection) was initiated along with methylprednisolone (16 mg/day), mycophenolate mofetil (500 mg twice per day) and hydroxychloroquine (200 mg twice per day). Methylprednisolone was slowly reduced. Surprisingly, hair regrowth in areas of alopecia on the scalp was observed after 6 weeks (Figure 1 B), with rash significantly relieved. After 24 weeks, the dose of methylprednisolone was tapered to 2 mg/day and the patient had prominent hair regrowth (Figure 1 C). Telitacicept had been well tolerated, and no adverse events were observed in the patient.
Figure 1
A – Alopecia areata involving front of the head before treatment. B – Hair regrowth in the alopecic patches after treatment with telitacicept for 6 weeks. C – Prominent hair regrowth after treatment with telitacicept for 24 weeks
SLE is a heterogeneous autoimmune disease, characterized by loss of immunological tolerance to self-antigens, aberrant T- and B-cell responses, and autoantibody production. Hair loss is commonly observed in SLE patients (20–60%), and there was a high incidence of alopecia areata in SLE patients [5, 6]. Alopecia areata is an autoimmune disease characterized by non-scarring hair loss and follicular preservation. Under some external triggers, such as infections, nutritional factors or psychological stressors, inflammatory cells, particularly CD8+ NKG2D+ T lymphocytes, cluster around hair follicles and produce large amounts of IFN-γ, leading to increased expression of MHC I on hair follicle epithelial cells. They bind to hair follicle peptides as non-self-antigens, and are further recognized and attacked by CD8βT cells, resulting in the formation of alopecia areata. B cells are also activated in alopecia areata and various autoantibodies have also been found in the sera [7]. B lymphocyte stimulator (BLyS/BAFF), a cytokine of the TNF ligand superfamily, can mediate the survival, proliferation, and maturation of B lymphocytes, and also activate T cells directly and may act as a Th1 response-promoting cytokine. It was reported that elevated IFN-γ in lesions of alopecia areata stimulates the production of BAFF and some alopecia areata patients had extremely high serum BAFF levels, which may induce the overall expansion of the alopecia areata immune response, including T cell responses. Increased BAFF production tended to correlate with worsened states of disease progression [8, 9]. Inhibition of the proliferation of B lymphocytes and block of BAFF may be a reasonable and specific strategy for the therapy of alopecia areata.
Telitacicept is a novel recombinant fusion protein of both transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and the Fc portion of human immunoglobulin G (IgG) (TACI-Fc). BAFF and a proliferation-inducing ligand (APRIL, another cytokine of the TNF ligand superfamily) receptors include TACI, B-cell maturation antigen, and B-cell-activating factor-receptor. Telitacicept can effectively block the proliferation of B lymphocytes and the maturation of T lymphocytes by binding and neutralizing BAFF and APRIL [10]. It is currently marketed in China for the treatment of adult patients with moderate to severe SLE. It has been proved to have good clinical results and appears to be well tolerated.
This study presents the inaugural use of telitacicept in treating a patient with SLE with alopecia areata, achieving favourable outcomes. The potential mechanism underlying telitacicept in the treatment of SLE with alopecia areata was preliminarily expounded. This finding will provide a new treatment approach for SLE with alopecia areata. However, more clinical studies are necessary to confirm this.