Amantadine is a well-known medication with indications in neurology and infectious diseases. It is currently Food and Drug Administraction (FDA) approved for Parkinson’s disease, drug-induced extrapyramidal symptoms, and influenza. In view of viral resistance, it is not currently recommended by the Centers for Disease Control and Prevention (CDC) for influenza. In the European Union it is approved by the European Medicines Agnecy (EMA) for the treatment of Parkinson’s disease and Parkinsonism.
In influenza A, the mechanisms of action of amantadine are thought to be related to the interference with the endosome by causing its alkalinization and disrupting the proton channels, thus interrupting the release of virions into the cell [1].
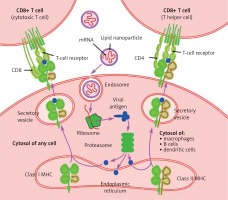
Lipid nanoparticles (LNP) have been used as a transport system for several medications since the 1990s, with applications in infectious diseases and oncology [2]. Recent developments have led to the application of LNP technology to deliver mRNA into host cells, particularly as part of new vaccines against SARS-CoV-2. The mechanism of mRNA incorporation into LNP is related to the cationic properties of LNP and anionic properties of mRNA, with subsequent formation of intracellular endosomes in order to release the mRNA into the matrix [3] (Figure 1).
COVID-19 is an infectious disease caused by the novel coronavirus SARS-CoV-2, which has rapidly developed into a world-wide pandemic. In the search for a drug therapy, observations have been made that nursing-home patients with neurological diseases such as multiple sclerosis, Parkinsonism, and cognitive impairment have milder COVID-19 than comparison groups, as reported by Rejdak et al. [4]. This has stimulated discussion about the need for further research, because no randomized controlled trials have been published on this topic to date [5].
On 3 Dec 2020, the British medicines agency MHRA gave the emergency-use authorization for the first vaccine based on the LNP-mRNA platform – BNT162b2. Since then, both the US FDA and EU EMA authorized for emergency use 2 vaccines based on LNP-mRNA technology: BNT162b2 and mRNA-1273. So far, no precautions for the use of the vaccine, as far as concomitant use of amantadine, have been published.
As more people are going to be vaccinated and more similar vaccines are going to be introduced, we should take into consideration the potential of amantadine to interfere with vaccine mRNA delivery into the target cells. The lipophilic properties of amantadine and its ability to interrupt the endosome [1, 6] suggest that it might interfere with successful release of the mRNA into the cell matrix, and subsequently with its binding to ribosomes.
This hypothesis warrants further studies; it is however important to point out such a possibility, especially that patients suffering from conditions treated with amantadine are among the first groups of vaccine candidates. A more cautious approach to these patients should be considered. Moreover, research on the effectiveness of amantadine as an antiviral therapy for COVID-19, and reports that patients have already been using this medication for COVID-19, could identify additional groups of patients, in whom potential problems with vaccine effectiveness may occur.