Introduction
Breast cancer is the most common worldwide malignancy in women, and its incidence is increasing due to the success of mammography screening programmes, which enable the detection of small and often non-palpable tumours [1]. At present, 25–35% of diagnosed breast cancer tumours are non-palpable [2]. As a result, techniques using markers for precise localisation of the tumour have been developed, with markers being introduced by a radiologist into the centre or to the periphery of the tumour, thus simplifying its detection during surgery.
Targeted axillary dissection (TAD) presents another important option in breast cancer surgery, where the employment of reliable markers is of paramount importance. Targeted axillary dissection consists of a sentinel lymph node biopsy (SLNB) and the excision of the pathological lymph node, which has been marked before neoadjuvant therapy. If metastasis is found in the sentinel lymph node or in a marked pathological node, axillary dissection (AD) levels I and II are performed. Targeted axillary dissection seems to be more accurate (by virtue of the false-negativity rate) than SLNB only, especially in women after neoadjuvant therapy with initially node-positive axillary status [3], although it is dependent on the reliability of the used marker.
Apart from standard markers in breast cancer surgery (such as wire localisation or metal clips), magnetic markers present a promising new option for breast tumour localisation. A literature search revealed that published data on the topic are highly insufficient, and so the aim of the present paper is to offer an up-to-date review focused on magnetic marker localisation in breast cancer surgery.
Non-magnetic markers
Many techniques are used nowadays for localising non-palpable breast tumours. Wire-guided localisation (WGL) is a widely used method, and it was first reported in 1965 [4]. The most commonly published disadvantages of WGL are patient discomfort, wire migration or transection, limitations to surgical incisions because of wire placement, vaso-vagal episodes and complications regarding surgery scheduling (WGL must be performed on the same day as surgery) [4–7].
Implanted tissue marker clips are markers without a specific detection system, and therefore preoperative localisation by WGL or a specimen radiograph (after excision of the lesion for confirmation that the clip is included) is necessary. Clip migration and problematic clip localisation present the main difficulties of the technique [8].
Carbon marking is a cheap marking technique that creates a tattoo in place of an injection, but it can imitate malignancy as a result of foreign-body giant-cell reaction [7, 9, 10].
Radioactive seed localisation (RSL) using iodine-125 (125I) seeds was first described in 1999 by Dauway et al. [11]. Since then, authors of several studies have demonstrated the non-inferiority of RSL compared to WGL [12–14]. The main advantage of RSL is that the seed can be put in place many days or even weeks before surgery, which allows for much easier surgery scheduling. The main RSL disadvantage rests in radiation safety regulations.
In the last few years, several new and non-radioactive non-wire localisation methods have appeared. SAVI SCOUT uses infrared light and radar technology, whereby the marker (12 × 4 mm with two 4 mm-long antennas) is detected by a handpiece and console system [5, 6]. Available data suggest that SAVI SCOUT is comparable to WGL in terms of both the negative margin and the re-excision rates. However, its main limitations are cost, nickel content relating to the risk of allergy reaction, device failure by interaction with electrocautery and high directionality of the system [5, 6].
Radiofrequency identification tags (RFIDs) are based on radio wave transmission and contain a microprocessor in which information can be stored [7]. It has a history of usage as an identification device, e.g. for pets, but the first clinical data about intraoperative use are very promising, with one advantage over other localisation technologies – the probe can detect distance from the tag [15, 16].
Magnetic markers
At present, there are two markers that use magnetic susceptibility to localise tumours in breast cancer surgery – Magseed and MaMaLoc (Table I).
Table I
Summary of magnetic localisation methods
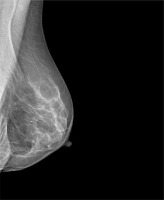
Magseed (Endomagnetics, Inc.) was approved by the FDA for breast lesion localisation in 2016 [6]. The method utilises 1 × 5 mm stainless steel magnetic seeds (Figure 1) implanted by an 18 G sterile introducer (Figure 2) under mammography or ultrasound guidance (Figure 3). After Magseed implantation, the marker position cannot be changed, which is similar to other non-wire markers [4]. During surgery, the seed is detected by a handheld magnetometer, SentiMag (Endomagnetics, Inc.) (Figure 4) at a distance up to 30 mm away [17, 18]. If the Magseed is placed deeper than 30 mm, it may not be detectable, but using palpation with a probe, which means tissue compression, deeper Magseeds are possible to detect. The Magseed may be implanted safely up to 30 days before surgery, but ongoing clinical trials are currently investigating a longer implantation period, which could be crucial in breast tumours or positive axillary lymph node localisation in patients after neoadjuvant therapy (NAC), which usually lasts 3–4 months. The main limitation of the Magseed system is noted when magnetic resonance imaging (MRI) is part of the treatment protocol, e.g. restaging after NAC. Although the Magseed is MRI-compatible, it has a bloom effect of up to 4–6 cm [5, 7], which renders radiological evaluation of the tumour in breast tissue unfeasible.
Figure 1
Magseed – 1 × 5 mm stainless steel magnetic localisation seed (with permission of Sysmex CZ Ltd)

Figure 3
Mammogram with Magseed implementation at the site of the breast tumour (University Hospital Ostrava)
To date, there are only four prospective studies and four conference abstracts offering clinical data regarding Magseed magnetic markers (Table II). The highest number of implanted seeds (73 seeds in 64 patients) was published by Price et al. [18], but data regarding Magseed migration are unfortunately lacking in this study. The other three prospective studies, working with 10, 29 and 32 implanted Magseeds, reported 0% seed migration. Moreover, the authors of all four studies reported 100% successful placement and detection of the seed in patients with various breast volumes [17–20]. Harvey et al. reported a correlation between breast size and the ease of detecting the Magseed, in that the detection time was shorter in smaller breasts, and the initial count on the SentiMag probe was higher [17]. Dorsal tumour localisation in large breasts may cause problems in detecting the Magseed, but the palpation of the seed may help with initial localisation. Unsuccessful detection of the Magseed has not been described in the literature to date.
Table II
Review of articles – Magseed system
Positive/negative resection margins present an extremely important criterion of localisation method reliability in breast cancer surgery. Negative margins are defined as clear margins without tumour cells or at a distance 1 or 2 mm away from the breast tumour, according to various authors [21, 22]. Markers are usually implanted into the centre of the tumour. Marker implantation and detection accuracy contribute to achievement of negative margins. Three prospective studies presented 0% positive margins in all patients with Magseed localisation [17, 19, 20]. The main limitation of these outcomes is a low number of included patients (10, 28 and 32 patients). Price et al., for instance, reported a positive resection margin in 12% of 64 patients with 73 seeds [18]. In comparison with other localisation methods, WGL has a negative margin rate of 70–88% [9], RSL 73.5–96.7% [5], clip markers 90–92% [23] and SAVI SCOUT 85.1–92.6% [5]. According to these first results, Magseed localisation seems to be comparable with other breast localisation markers in terms of oncosurgical radicality. A prospective comparative study between Magseed and other markers is currently lacking.
The Magseed system can be used to localise multiple lesions in the same breast. The limitation is the proximity of lesions under 2 cm, which may result in the inability of the magnetometer to separate signals [18]. However, it is questionable whether it is actually necessary to separate signals emanating from two close lesions during breast-conserving surgery, because for negative margin achievement, surgeons need to excise a sufficient amount of the surrounding tissue.
The interference of the Magseed signal with electrocautery or paramagnetic surgical instruments is another very important limitation of magnetic marker localisation techniques [4, 6, 9, 24]. Non-conductive instruments (i.e. polymer or carbon fibre) need to be used while scanning with a Sentimag probe [5], but it is not necessary to use non-conductive instruments during the entire surgical procedure. In case of interference with magnetic instruments, the Sentimag probe needs to be recalibrated [6]. The frequent need for recalibration is one of the biggest drawbacks of the Sentimag system.
The localisation of pathological axillary nodes for targeted axillary dissection (TAD) by means of a Magseed system presents another very promising option in breast cancer surgery [17]. According to NCCN guidelines, TAD is a possible option in staging axillary status after neoadjuvant treatment in patients with initially metastatic axillary nodes. Using only SLNB, the false-negativity rate stands at over 10% in this group of patients, which is unacceptable, especially when one recognises that, in comparison, TAD has a false-negativity rate of 2% [3]. Marking of the pathological node in axilla is dependent on a safe and reliable marker and its localisation system. According to the literature, various localisation methods are used – RSL [3], clip markers [25] or carbon marking [26], each with its own advantages and disadvantages as discussed above. Magseed properties such as no marker migration and intuitive detection [17, 19] could be crucial in this area, but no clinical study has been published on this topic in the field of breast cancer surgery, although two prospective open-label studies are currently ongoing at the M.D. Anderson Cancer Center (NCT03038152) [27], (NCT03796559) [28].
Two prospective studies (Pohlodek et al. 2018, Hersi et al. 2018) have investigated the simultaneous use of Magseed and SLNB with the magnetic tracer Sienna (Endomagnetics, Inc.) [19, 20], which is a solution containing superparamagnetic iron oxide nanoparticles that are injected into the breast pre-operatively and accumulate in the sentinel lymph node through lymphatics, before detection by a Sentimag probe [19]. The authors found no interference between these two magnetic methods – Magseed for breast lesion localisation or Sienna for SLNB – when using the same Sentimag probe for detection [19, 20], and so they concluded that a combination of Magseed and Sienna should reduce the use of radioactive methods in breast cancer surgery [19, 20]. The successful separate usage of Sienna for SLNB has been reported in prospective studies since 2014 [29–31]; moreover, in 2016, the first meta-analysis was done by Karakatsanis et al., indicating that the effectiveness of the Sienna system is comparable to standard techniques using a radioactive tracer [32].
The main drawback of Magseed is the cost of the system, in that the price of the Sentimag probe and each implanted magnetic seed is much higher in comparison to wire-guided localisation [17, 20]. Harvey et al. proposed that a full cost analysis (considering factors such as faster scheduling in the operating theatre, reduced restrictions due to radiation safety policy and patient satisfaction) could balance out the higher Magseed price [17].
The MaMaLoc (magnetic marker localisation) system, which is another magnetic marker used for breast tumour localisation, was developed at The Netherlands Cancer Institute [33]. The only prospective single-centre study regarding the MaMaLoc system (Figure 5), for which 15 female patients with non-palpable breast cancer tumours for surgical therapy without NAC were recruited, was published in 2017 [33]. All MaMaLoc markers were placed successfully 5–30 days before surgery, and a radioactive 125I seed reference marker was also placed in each patient. MaMaLoc migration ranged from 0 mm to 0.5 mm, which is clinically irrelevant, because it does not change the site of operation. MaMaLoc detection was provided by a Sentimag probe (Endomagnetics, Inc.). The identification rate for the marker during breast-conserving surgery was 100%, and all tumours were excised. The study does not provide evidence about positive/negative resection margins of excised tumours, but these criteria are scheduled for assessment in an ongoing MaMaLoc-2 trial (NTR6767) [34].
Figure 5
MaMaLoc marker – 1.5 × 3.5 mm magnetic localisation marker (with permission of Sirius Medical Systems B.V.)

MaMaLoc is not compatible with MRI. Because the Sentimag probe is used, there is a recommendation as in Magseed to use non-conductive tools during the surgery whilst detecting magnetic markers. MaMaLoc technology is not currently commercially available, but The Netherlands Cancer Institute has created a spin-off company to spread the MaMaLoc system worldwide. Magseed is commercially available in many countries in Europe, North America, Asia, Africa and Australia.
Conclusions
Magnetic marker localisation is a safe and reliable method for breast tumour marking. There are four prospective studies available, each of which demonstrates that the Magseed system has a negative margin rate and a successful localisation rate comparable to standard marking systems used in breast cancer surgery. The main benefits of magnetic markers are that they require no radiation safety measures, and they offer the possibility of longer deployment times, thus simplifying surgery scheduling. The most important drawbacks are the cost of the system, depth limitation and need for frequent probe recalibration.