Introduction
Esophageal squamous cell carcinoma (ESCC) is a life-threatening type of skin cancer occurring worldwide including China. So far, radical surgery is a valid therapeutic method. However, the prognosis is still unsatisfactory [1, 2]. The ESCC patients’ 5-year survival rate with pathologic lymph node negative metastasis (pN0) could be nearly 70%, but some of the patients still suffer tumor recurrence [3, 4]. Lymph node metastasis (LNM) and lymph node micrometastasis (LNMM) are prognostic factors in ESCC [5]. LNM and LNMM molecular mechanisms in ESCC are not completely clarified. Tumor TNM stage is commonly used in assessing tumor patients’ prognosis, but most of the time we found that TNM stage was not in conformity with the actual prognosis.
With the development of molecular biotechnology, some new ways have been used in tumor prognostic research. Mucin 1 is a cell surface glycoprotein and has been regarded as an epithelial tissue specific marker. Mucin 1 is observed on normal glandular epithelial cells and its number could be significantly increased when the cells become malignant [6, 7]. Some research has shown that mucin 1 was related to poor prognosis of some carcinomas [8–11]. Recently it has been reported that mucin 1 is associated with LNMM, and could exactly be used to estimate LNMM of lung cancer patients who underwent radical surgery [12].
The purpose of our study is to assess whether mucin 1 expression detected by immunohistochemistry in lymph nodes correlates with LNM, LNMM and prognosis in patients with ESCC.
Material and methods
Patients
A total of 92 patients were enrolled in the research, at the Department of Thoracic Surgery, Jinan Central Hospital from January 2009 to December 2013. The eligibility standards were as follows: (1) ESCC was confirmed by curative resection and postoperative pathology for all the subjects. (2) No cervical or supraclavicular lymph node metastasis was found by cervical B ultrasound or preoperative computed tomography (CT). (3) No serious contraindications were found. (4) TNM staging (the 8th edition) was referred to the International Union Against Cancer (UICC) [13]. This research was approved by the Jinan Central Hospital’s Ethics Committee.
Samples and Immunohistochemistry
Overall, 1382 lymph nodes were obtained from the 92 patients in the experimental group. A total of 20 paraesophageal lymph nodes were obtained from 10 benign esophageal disease cases as the control group. To avoid sample heterogeneity, 3 mm thick sections at each level from each paraffin block were used. The first slice was stained with HE. The other was stained with immunohistochemistry. The HE-stained slices were carried out on paraffin-embedded and immunohistochemical reactions. Primary antibodies against mucin 1 (dilution 1 : 100, Maxim Inc., Fuzhou, China) were used. The immunostained slides were independently evaluated by two of our authors. The diagnostic criteria of LNMM were as below: (1) No tumor cells were observed on the HE stained slices. (2) At least one strongly immunoreactive epithelial cell in the cortex of the lymph node or in the subcapsular sinus was observed ranging from 0.2 mm to 2 mm on the immunohistochemical slices.
Follow-up
The subjects underwent routine examination every 3 months. They received physical examination, chest and abdomen CT, and some of them were also examined by positron emission tomography combined with CT (PET/CT).
Statistical analysis
The χ2 test or Fisher’s exact probability test was used to analyze the enumeration data. The Kaplan-Meier method was used to analyze the survival results. The Cox proportional hazard model was used for multivariate analysis. P-values less than 0.05 were considered significant. The statistical data were processed with the SPSS 23.0 software.
Results
Mucin 1 expression in lymph nodes
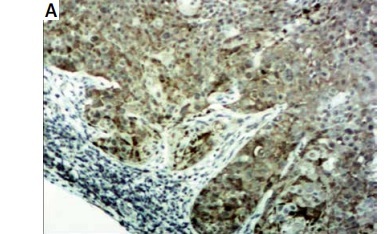
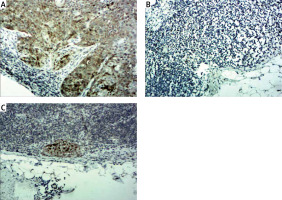
Mucin 1 was detected in 1382 lymph nodes from 92 patients (Figure 1). In the pN1-2 patients’ group, 68 lymph nodes from 15 patients had tumor metastasis. All the 68 tumor metastatic lymph nodes were positive for mucin 1. The diagnostic sensitivity was 100% (68/68). Mucin 1 was detected in another 231 lymph nodes and among them, 3 (3/231 1.3%) lymph nodes from 2 (2/15 13.3%) patients were positive for mucin 1. In the 77 pN0 patients, mucin 1 was detected in 1083 lymph nodes from the 77 patients; 17 (17/1083 1.6%) lymph nodes from 15 (15/77 19.5%) patients were positive for mucin 1. In the control group, mucin 1 was detected in 20 lymph nodes from the 10 patients. No mucin 1 positive case was observed in the lymph nodes. The above data show that mucin 1 expression in the pN1-2 group was significantly higher than that in the pN0 group (p < 0.01), and was also higher than that in the control group (p < 0.01). There was no significant difference between mucin 1 expression in the pN0 patient group and the control group (p > 0.05).
Correlation between mucin 1 expression and prognosis
After surgery, 61 patients accepted chemotherapy, and 31 patients accepted radiotherapy. The 92 patients’ 5-year survival rate was 39.1%, which was significantly related to tumor invasion (pT, p < 0.05), lymph node metastasis (pN, p < 0.01), pTNM stage (p < 0.01) and mucin 1 expression (p < 0.01) (Figure 2, Table I). The 5-year survival rate of the patients with mucin 1 expression in lymph nodes group was significantly lower (10.0% vs. 53.2%; p < 0.01) than in the group without mucin 1 expression. Cox regression of multivariate analysis demonstrated that mucin 1 expression and pT were independent prognostic factors (Table II).
Table I
Correlation between 5-year survival and clinical features of the 97 patients with esophageal squamous cell carcinoma
Table II
Results of Cox regression multivariate survival analysis
Figure 2
A – Kaplan-Meier analysis of overall survival. B – Kaplan-Meier analysis of overall survival rate in patients with pT. C – Kaplan-Meier analysis of overall survival rate in patients with pN. D – Kaplan-Meier analysis of overall survival rate in patients with pTNM stage. E – Kaplan-Meier analysis of overall survival rate in patients with mucin 1 expression

Discussion
Being a transmembrane glycoprotein, mucin 1 is observed in many epithelial cell types [14]. It participates in cell adhesion modulation and protection from infection [15, 16]. Furthermore, mucin 1 could be overexpressed in some kinds of tumors and prevent immunocytes from attacking cancer cells [17]. Previous studies have reported that mucin 1 expression was related to poor prognosis in some cancers [18–21]. A similar tendency has been reported for ESCC [22]. Our previous study showed that both mucin 1 mRNA and protein expression in ESCC tissue was related to tumor invasion, lymph node metastasis, pTNM stage and poor prognosis for ESCC patients [23]. In this study, we investigated mucin 1 expression in lymph nodes in ESCC patients who accepted radical resection using immunohistochemistry. In the pN1-2 patient group, 68 lymph nodes from 15 patients had tumor metastasis. All the metastatic lymph nodes showed mucin 1 expression. None of the mucin 1 was detected in the benign esophageal disease group. Our results provided evidence at the protein level that mucin 1 might be a biomarker for detecting LNM in ESCC.
In this study, we also found mucin 1 expression in the pN0 patient group. It implied that there existed LNMM which was not detected by pathology. So far, no definite gene could predict LNMM. The common markers used to detect LNMM include cytokeratin (CK), carcinoembryonic antigen (CEA), E-cadherin and matrix metalloproteinase (MMP) [24]. The standard diagnostic method of LNMM needs one or a few histopathologic HE stain sections from each lymph node. However, previous studies showed that about 20% of LNMM could not be detected with this method [25]. LNMM is defined as a small metastatic lesion measuring from 0.2 to 2 mm tumor cells in a lymph node, which could not be detected by conventional pathology methods [26]. Several methods of detecting micrometastases are available, including reverse polymerase chain reaction (PCR) and immunohistochemical staining.
Up to now, continuous pathological section has been the most accurate diagnostic method for LNMM. However, it is time consuming and complex, so it could not be routinely used in many hospitals. PCR has been used for detecting LNMM. Sensitivity is the greatest superiority of this method. PCR could detect a tumor cell in 1 × 107 cells [27]. Moreover, even poorly differentiated tumors with tissue-specific proteins unexpressed could also be detected for tumor mRNA expression using the PCR technology. PCR also has a shortcoming: contamination or the presence of a pseudogene might lead to false positives. Target markers’ heterogeneous expression could also cause false negatives. Up to now, pathologic diagnosis is still the gold standard. When using PCR to detect LNMM, a lymph node should be divided in half with one half for PCR and the other for routine HE. However, when the tumor is located in only one half of a lymph node specimen, it could affect the accuracy of the PCR result [27]. Immunohistochemistry could also improve the LNMM detection. It uses monoclonal antibodies with specific anti-tumor cell associated antigens with chromogenic agents which respond to the lymph node antigen. It can also show both morphological and functional features. With accurate localization and high sensitivity, immunohistochemistry could be widely used in clinical practice to detect LNMM. Immunohistochemistry also has its limitations. It is not suitable for cases with loss of antigen expression in poorly differentiated tumors. It can also be susceptible to the subjective readout of immuno-stained cells. Due to the above reasons, the validity of the diagnosis for LNMM should be confirmed by multi-institutional studies using different analytic methods.
Salerno was the first to detect mucin 1 mRNA expression in lymph nodes by RT-PCR and found that mucin 1 mRNA can diagnose LNMM in lung cancer [28]. The sensitivity of mucin 1 detection in diagnosing LNMM was as good as CK as reported in the literatures [29]. Our previous study showed that mucin 1 mRNA can diagnose LNMM in ESCC patients by RT-PCR and the diagnostic sensitivity was 28.1% [30]. In this study, in the group of 77 pN0 patients, mucin 1-positive carcinoma cells were found in 17 (1.6%) of 1083 lymph nodes from 15 (19.5%) patients, showing that LNMMs were found in 15 patients with 17 lymph nodes. Our study showed that mucin 1 could diagnose LNMM in ESCC patients by immunohistochemistry and progression from stage I–IIA to IIB–III in some cases.
Previous studies have shown that tumor relapse and poor survival after surgery were correlated with the LNMM detected by IHC or RT-PCR [3–5]. Our data were consistent with their conclusion. The 97 patients’ 5-year survival rate was significantly related to tumor invasion (pT), lymph node metastasis (pN), pTNM stage and mucin 1 expression. Cox multivariate regression analysis was performed to eliminate the impact of mixed factors related to prognosis on statistical analysis, and the results showed that mucin 1 expression and pT were independent relevant factors.
In conclusion, mucin 1 expression was related to LNM, LNMM and poor prognosis in ESCC patients. Immunohistochemistry for mucin 1 can be applied for the detection of LNM and LNMM. Our data demonstrated that mucin 1 was a prognostic marker as well as a molecular target for the effective treatment of ESCC patients.