Introduction
Colorectal cancer is one of the most commonly diagnosed cancers in the world [1, 2]. Rectal cancer accounts for nearly one third of the total incidence of colorectal cancer [3, 4]. The inferior mesenteric artery (IMA) is one of the three unpaired branches of the abdominal aorta, which supplies the left side of the colon and rectum. It arises from the anterior aspect of the aorta below the renal artery branch points accompanied by the inferior mesenteric vein (IMV) [5]. For rectal cancer, the root of the IMA should be ligated in a standard procedure of total mesorectal excision [6, 7]. Although several previous studies have shown a significantly increased IMV diameter in patients with rectal cancer [8, 9], no study has yet reported the IMA enlargement in rectal cancer. Thus, this study aimed to assess the association between the IMA diameter and rectal cancer.
Material and methods
Patients
The current study was approved by the ethics committee of Taizhou Hospital of Zhejiang Province, and all study participants provided informed consent. This study included a consecutive series of patients with primary rectal cancer in our center from July 2017 to June 2019. All patients underwent contrast enhanced computed tomography (CT) of the abdomen and pelvis after the presence of rectal cancer was confirmed by colonoscopic biopsy. Baseline patient characteristics, such as age, sex, weight, body mass index (BMI), and TNM stage (7th AJCC classification), were retrospectively retrieved [10]. Patients were excluded if they had neoadjuvant treatment, synchronous colon cancer, history of abdominal surgery, and inflammatory bowel disease or severe atherosclerosis of the abdominal aorta branches, as these conditions might alter the IMA diameter. A control group of 42 patients with abdominal pain without rectal cancer or pathology in the IMA territory who underwent contrast enhanced CT of the abdomen and pelvis in the same time period was also selected.
Imaging protocol and measurement of the inferior mesenteric artery diameter
All patients underwent contrast enhanced CT of the abdomen and pelvis on a 64-slice Toshiba Aquilion CT scanner using a standardized protocol. They were injected intravenously with 90 ml of iopromide (370 mg/ml, Iopamiron 370; Bayer, Osaka, Japan) at a speed of 3.0 ml/s. Abdominal and pelvic scans were obtained using the following parameters: 120 kVp, 220 mAs, and 0.5 mm slice thickness. The multiphase CT scanning protocol included unenhanced, arterial (30 s delay), portal venous (60 s delay), and equilibrium phases (120 s delay).
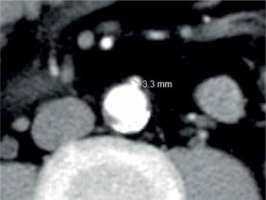
The arterial phase axial CT images were used to assess the IMA diameter. A single observer performed the measurements at the IMA origin using an electronic caliper on an imaging workstation where the images were magnified to 400% to increase accuracy (Figure 1). A second observer who was blinded to the former observer’s results performed the measurements in the same way to confirm intra-observer agreement and reproducibility.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables and as a number and percentage of the total for categorical variables. Student’s t-test and the Mann-Whitney U test were applied to evaluate continuous variables, and the χ2 test was performed to analyze categorical variables. The one-way analysis of variance and the least significant difference test were used for comparing continuous variables in multiple comparisons. The relationship among variables was assessed using the Pearson correlation coefficient. A p-value of less than 0.05 was used as the level of significance. All statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM Co., Armonk, NY, USA).
Results
In the period from July 2017 to June 2019, 150 patients were newly diagnosed with rectal cancer. Eleven patients receiving neoadjuvant treatment, 2 patients with a synchronous colon cancer and 2 patients with a history of abdominal surgery were excluded. Finally, 135 patients were enrolled in the patient group, 89 of whom were men and 46 women. Of the 135 patients, 21 patients who had presence of metastasis (M1) were classified as having stage IV cancers, while 114 patients who had absence of metastasis (M0) were adopted for surgical resection. Among the 114 surgical patients, according to the postoperative pathology, there were 28 patients with stage I cancers, 40 patients with stage II cancers, and 46 patients with stage III cancers. There were 4 patients with T1, 31 with T2, 51 with T3, and 28 with T4 tumors. Seventy-eight patients had N0 tumors, and 46 patients had lymph node involvement (N1 = 34, N2 = 12). Forty-two patients were in the control group, with 18 women and 24 men.
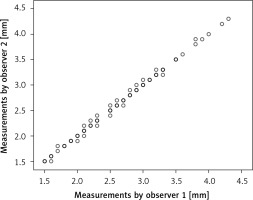
There was no significant difference in sex, age, weight, or BMI between the patient group and control group. However, we found that the mean IMA diameter was wider in the rectal cancer patients (2.49 ±0.53 mm) than in the control group (2.20 ±0.47 mm), which was statistically significant (p < 0.001) (Table I). There was an excellent correlation between the two observers in measuring the IMA diameter (r = 0.998, p < 0.001) (Figure 2).
Table I
Baseline patient characteristics
Figure 2
Intra-observer correlation plot for measurement of the inferior mesenteric artery (IMA) diameter. The correspondence between two observers was nearly perfect (r = 0.998, p < 0.001)
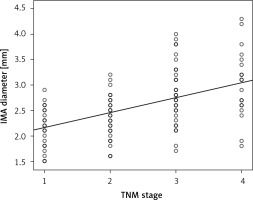
The IMA diameter of patients with stage I, stage II, stage III, and stage IV cancers was 2.24 ±0.36 mm, 2.45 ±0.39 mm, 2.80 ±0.55 mm, and 2.85 ±0.51 mm, respectively (p < 0.001). Pearson analysis revealed a moderate, statistically significant linear relationship between IMA diameter and TNM stage (r = 0.519, p < 0.001) (Figure 3).
Figure 3
Correlation between TNM stage and inferior mesenteric artery (IMA) diameter. Pearson analysis showed a positive and moderate correlation between TNM stage and IMA diameter (r = 0.519, p < 0.001). TNM stages 1, 2, 3, and 4 (X-axis) represent TNM stages I, II, III, and IV, respectively
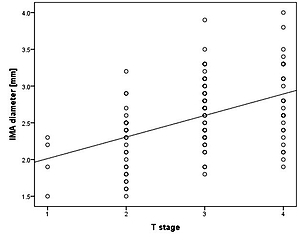
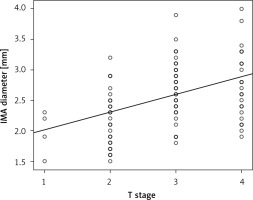
The IMA diameter of patients with T1, T2, T3, and T4 tumors was 2.18 ±0.31 mm, 2.39 ±0.50 mm, 2.55 ±0.48 mm, and 2.73 ±0.51 mm, respectively (p < 0.001). A moderate, statistically significant linear relationship was also found between IMA diameter and T stage (r = 0.457, p < 0.001) (Figure 4).
Figure 4
Correlation between T stage and inferior mesenteric artery (IMA) diameter. Pearson analysis showed a positive and moderate correlation between T stage and IMA diameter (r = 0.457, p < 0.001)
The IMA diameter of patients with N0, N1, and N2 tumors was 2.37 ±0.39 mm, 2.83 ±0.60 mm, and 2.71 ±0.40 mm, respectively (p < 0.001). However, we did not find a significant correlation between IMA diameter and N stage (r = 0.166, p = 0.077). Although patients with N1 and N2 tumors have a wider IMA diameter than patients with N0 tumors (N0 vs. N1, p < 0.001; N0 vs. N2, p = 0.020), there was no significant difference between patients with N1 and N2 tumors (p = 0.448). The IMA diameter of patients with M1 tumors was significantly wider than that in patients with M0 tumors (M0 = 2.54 ±0.51 mm vs. M1 = 2.85 ±0.51 mm, p = 0.011).
Discussion
To our knowledge, this is the first study to investigate the relationship between the IMA diameter and rectal cancer. Our findings reveal that the IMA in rectal cancer patients enlarges as the TNM stage gets higher. The IMA diameter could be a potentially important marker for the staging of rectal cancer.
For rectal cancer, the optimal treatment strategies should be developed according to the stage of disease at patient presentation [11]. Patients with favorable prognostic features can proceed to total mesorectal excision directly. However, patients with poor prognosis tumors or metastasis usually require neoadjuvant chemoradiation therapy or palliative chemotherapy [12]. Magnetic resonance imaging (MRI) is the most accurate method to evaluate the local tumor extent of rectal cancer because of its high soft tissue contrast resolution [13]. MRI allows the precise assessment of the tumor relationship to the mesorectal fascia, which also defines the circumferential resection margin involvement in total mesorectal excision surgery [14]. Computed tomography is useful for systemic staging of rectal cancer, because it can examine the thorax, abdomen, and pelvis in a single examination. For evaluating the primary tumor, noninferiority of CT, as compared with MRI, has been reported in several studies [15, 16]. Additionally, CT is cheaper and more time-saving than MRI. For these reasons, CT has become a good alternative method for the staging of rectal cancer and influences the therapeutic approach to patients [17]. In our hospital, CT of the thorax, abdomen, and pelvis is routinely performed in rectal cancer patients. In the present study, 15 patients were identified as requiring tumor downstaging on CT images and received neoadjuvant therapy. Distant metastases were identified in 21 patients. The remaining 114 patients underwent total mesorectal excision successfully.
Improvement in CT technologies has not only enabled accurate and non-invasive diagnoses of rectal cancer but also helped evaluate correlations between rectal cancer and vascular abnormity (increased blood flow and dilated blood vessels) [18]. Recently, changes in venous circulation have been observed in patients with colorectal cancer. Wu et al. reported that the IMV is significantly dilated in rectal cancer [19]. Khan et al. found a link between the diameter of the superior mesenteric vein and right colonic cancer [20]. Conventional angiography was commonly regarded as the gold standard for evaluating the IMA. However, with development in CT technologies, three-dimensional CT angiography has been suggested as an alternative modality for evaluating the IMA [5]. In fact, the IMA can be excellently visualized even on routine CT examinations. In our study, using a standard process, we managed to measure the IMA diameter precisely on the arterial phase axial CT images. As a result, the two observers showed perfect correlation in measurement.
As expected, our results showed that the IMA diameter of patients with rectal cancer was significantly wider than in those without rectal cancer. We further analyzed the IMA diameter of patients with different TNM stages and found that IMA diameter positively and moderately correlated with TNM stage (r = 0.519). Additionally, we found a positive and moderate relationship between IMA diameter and T stage (r = 0.457). Although we did not observe a correlation between IMA diameter and N stage, we found that the IMA diameter of the patients with N1 and N2 tumors was significantly larger than that of patients with N0 tumors, and the IMA diameter of the patients with M1 tumors was significantly larger than that of patients with M0 tumors. The main finding of these results is that the IMA diameter increases as the staging of rectal cancer gets higher. This finding suggests that the IMA diameter is a potential auxiliary marker for the staging of rectal cancer, which is important, because additional information for the staging of rectal cancer can benefit patients in better identifying whether they need neoadjuvant treatment [21].
The possible mechanism for our favorable results is believed to be angiogenesis [22]. It is well known that angiogenic chemical substances secreted by tumors lead to the formation of new vessels to supply tumors with oxygen and nutrients for their relentless growth [23–25]. The IMA dilatation may also be triggered by these substances directly. In the process of angiogenesis development, the IMA could enlarge to accommodate the increased blood flow caused by the increased number of tumor vessels. It is important to evaluate tumor angiogenesis because it is known to play an important role in tumor invasion and metastasis [26]. Currently, intratumoral microvascular density assessed by immunohistochemistry in archival tissue is a commonly used method to qualify the intensity of tumor angiogenesis [27]. An association between high microvascular density and poor prognosis in colorectal cancer has been reported by several studies using this method [28, 29]. However, this method is limited to surgical patients whose resection specimens are available and cannot be used to predict or evaluate the response to neoadjuvant therapy or antiangiogenic therapy. Taking the above considerations and our findings together, we speculate that the IMA diameter has potential as a marker for prediction and evaluation of the effect of neoadjuvant treatment and antiangiogenic treatment.
The present study also has several limitations. First of all, this is a retrospective study, so there might have been selection bias. Furthermore, the samples are taken from a single center and thus are not large enough. The low number of patients with stage T1 is another limitation. Additionally, the measurement error is also an important limitation. However, in our study, we used a standard process of measurement to minimize measurement error. We have demonstrated that the measurement results were reproducible.
In conclusion, our study provides an important finding that the IMA in rectal cancer patients enlarges as the TNM stage gets higher. The finding suggests that the IMA diameter can be considered as a potentially important marker for the staging of rectal cancer. However, future well-designed prospective studies to aid in evaluating this relationship are recommended.