Introduction
Caesarean section (CS) is the most common interventional procedure performed in women all over the world. Although caesarean section can be a life-saving intervention for mothers and children, it can also lead to short-term and long-term health consequences.
The placenta is the interface between maternal and foetal circulations, integrating maternal and foetal signals to selectively regulate nutrient, gas, and waste exchange, as well as secrete hormones. In turn, the placenta helps create an in utero environment and controls foetal growth and development. The placenta differentiates from the extraembryonic trophectoderm, giving rise to villous cytotrophoblasts that can proliferate or fuse to form the multinuclear syncytiotrophoblast layer that acts as the barrier between the maternal and foetal circulations, and to extra-villous trophoblasts, which infiltrate the decidua and remodel the maternal spiral arteries [1]. This differentiation involves changes in gene expression mediated by distinct epigenetic alterations. By the blastocyst stage, the genome is almost completely hypomethylated; however, the imprinted epigenetic marks remain intact during this stage. At the blastocyst stage, the inner cell mass and trophectoderm undergo a wave of de novo DNA methylation but to different extents, resulting in differences in final global DNA methylation levels [2, 3].
Evidence suggests that the placental epigenome plays key roles in healthy foetal development, and all situations leading to changes in its global methylation may limit its functions [4, 5]. There is a hypothesis that a caesarean section changes the placental DNA methylation and has been linked to late infant health outcomes [6, 7]. Epidemiological and clinical studies have shown that children delivered by caesarean section are associated with a higher risk of developing illnesses such as asthma [8], respiratory disorders [9], and an increased risk of diseases associated with immune function [10]. However, a meta-analysis only identified an increased risk of obesity up to the age of 5 years, and asthma up to the age of 12 years in children born by caesarean section [11]. The elective caesarean section may increase the risk of child asthma due to exposure to different microbiota during delivery [12].
Epigenetics mechanisms, often defined as regulating gene activity independently of underlying DNA sequence, have been implicated in the development of many human diseases, including cancers [13], neurological disorders [14], type 2 diabetes [15], and hypertension [16]. The most studied epigenetic mechanism is DNA methylation, which involves the addition of a methyl group to a cytosine, usually in the context of cytosine-phospho-guanine dinucleotides (CpG site). DNA methylation is commonly associated with gene silencing and contributes to X-chromosomal inactivation, genomic imprinting, and transcriptional regulation of tissue-specific genes during cellular differentiation [17]. CpG sites often cluster together in CpG islands close to the promoter and regulatory regions of genes, and within this context increased DNA methylation blocks access to the underlying DNA sequence, leading to reduced gene expression [18]. Although CpG methylation status is conferred early in development and is relatively stable [19], it can change due to environmental exposure and lifestyle factors, e.g smoking [20, 21], chronic stress [22], or maternal disease [23]. The majority of these factors have been shown to affect both global and gene-specific placental methylation [24]. Moreover, uteroplacental insufficiency, the most common cause of foetal growth restriction, induces epigenetics modifications in placental cardiometabolic genes in which differential methylation may confer increased risk of cardiologic and metabolic disease in adulthood [25].
None of the placenta studies explores in detail the association between the mode of delivery, uterine contractions, and DNA methylation modification. Some hypotheses describe surges of stress hormones during labour that advance the development of essential pathways to enable the infant to survive outside the womb, thus implicating changes in the genome in response to stress. However, these changes may not occur if the infant does not undergo such stress, such as during a caesarean section.
In this study, we set out to characterise the relationship between caesarean section and DNA methylation patterning in the placenta. The measurement of global DNA methylation patterns is a practical means of identifying differential epigenetic effects in neonates. However, the measurement of global methylation cannot rule out differences on the single gene level. This is a pilot study, and in the future we are going to select a panel of genes responsible for obesity and immunomodulation. Moreover, we are going to finish the neonatal follow-up, and the results will be published.
Material and methods
The study was conducted in 2014–2017 at the delivery units of the First Department of Obstetrics and Gynaecology, Centre of Postgraduate Medical Education in Warsaw, and comprised 111 Caucasian pregnant women who were recruited consequently and fulfilled inclusion criteria for the study.
Prior to delivery, the pregnant women were requested to complete a questionnaire and were asked for permission to collect placental samples after detachment for research purposes. The participants were asked to provide information about their date of birth, race, height, pre-pregnancy weight, gestational weight gain, gestational age, parity, pre-existing and existing diseases, infectious diseases, use of antibiotics, and smoking, drug, and alcohol consumption. The inclusion criteria for the study were as follows: maternal age of at least 18 years, singular pregnancy, term delivery (gestational age at least 37 weeks), and newborn weight of at least 2500 g. The exclusion criteria from the study were: multiple gestation, maternal disorders such as preeclampsia, hypertension, diabetes, or gestational diabetes mellitus (GDM), asthma, smoking and drinking alcohol during pregnancy, illicit drugs, infectious diseases, renal disease, allergy, cognitive disorders, use of antibiotics, and premature rupture of membranes.
The foetal and newborn exclusion criteria from the study were as follows: intrauterine grown restriction, Apgar score less than 7 at 1st and 5th min, (Neonatal Intensive Care Unit (NICU) admission, malformations, or chromosomal disorders. Moreover, none of the pregnancies resulted from assisted reproductive technology. Deliveries with labour induction were not included in this study.
The study involved global methylation in placental tissue from 49 pregnant women, delivered by elective caesarean section (ECS) before the start of labour. In 16 cases, elective caesarean section regular uterine contractions were declared. This group was termed the intrapartum caesarean section group (ICS). Indications for all the CS included: previous CS, breech position, or pelvic disproportion. None of the caesarean sections were performed on the grounds of foetal distress and prolonged labour. A reference group was formed, consisting of 46 healthy pregnant women delivering by vaginal route (VD).
Spinal analgesia was applied in all cases in the elective and intrapartum caesarean section group. Women who underwent general analgesia were excluded from the study. In the vaginal delivery group, women with regular and painful uterine constrictions were offered epidural analgesia.
Full-thickness placental tissue samples were collected within 45 min of birth. All biopsies were performed by aseptic technique. A triangular segment of the placenta was cut near the cord insertion site. The DNA methylation levels were quantified using a 5-mC DNA ELISA kit.
This study was approved by the Centre of Postgraduate Medical Education Ethics Committee number 8/PB/2014.
Global DNA methylation assay
Full-thickness placental tissue was collected immediately after spontaneous, non-assisted delivery. Each placenta was sectioned transversally (1 cm) using a sterile scalpel near the cord insertion site. The placental sample was washed in sterile 1× phosphate-buffered saline (PBS, pH 7.5) to remove any residual blood and was immediately frozen in liquid nitrogen and stored at –80°C for DNA analysis. The Extract Me DNA Tissue kit (Blirt S.A., Poland) was used for DNA extraction and purification from the placenta. Qubit 2.0 (Thermo Fisher Scientific) was used to determine the DNA yield. Global DNA methylation was isolated from the placental tissue using the 5-mC DNA ELISA kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s instructions. Briefly, 100 ng of DNA was diluted with 5-mC coating buffer and incubated at 98°C for 5 min. After denaturation, the DNA was transferred to the plate and incubated at 37°C for 1 h. The samples were incubated with capture and detection antibodies, and absorbance was read at 450 nm. A quantification of global DNA methylation was obtained by calculating the amount of methylated cytosine (5-mC) in the sample relative to global cytidine (5 mC + dC) in the standard curve of a positive control that had been methylated previously. All samples were analysed in duplicate. The mean value per sample was used for further calculations.
Statistical analysis
The data were statistically analysed using Statistica 13 software. The minimum and maximum values, and the median and interquartile range (lower (25%) and upper (75%) quartiles) were estimated for continuous variables, as well as absolute numbers (n) and percentages (%) of the occurrence of items for categorical variables. The statistical tests were used as follows: Kruskal-Wallis’s H test – to compare continuous variables between three modes of birth; χ2 test – to compare categorical variables between three modes of birth; Mann-Whitney’s U test – to compare global DNA methylation between two modes of birth in pairs, between male and female newborns, as well as between epidural analgesia and no analgesia in vaginal deliveries; and Spearman’s r correlation coefficient – to correlate global DNA methylation with continuous characteristics. A significance level of 0.05 was assumed throughout the study.
Results
The maternal and gestational age, parity, pre-pregnancy and pregnancy body mass index (BMI), weight gain during pregnancy, newborn weight, height and gender, and Apgar score both at 1 and 5 min did not differ significantly between the elective and intrapartum caesarean sections and the vaginal delivery groups (Table I).
Table I
Clinical characteristics of the study group
| Characteristics | IU | Parameter | Elective caesarean section (N = 49) | Intrapartum caesarean section (N = 16) | Vaginal delivery (N = 46) | P-value# |
|---|---|---|---|---|---|---|
| Maternal age | years | Me (25–75%) | 32 (30–36) | 30 (28–36) | 30 (28–33) | 0.085 |
| Min.–max. | 19–43 | 25–41 | 24–40 | |||
| Gestational age [weeks] | 37–38 | n (%) | 13 (26.53) | 5 (31.25) | 11(23.91) | 0.844 |
| 39–41 | n (%) | 36 (73.47) | 11 (68.75) | 35 (76.09) | ||
| Parity | 1 | n (%) | 22 (44.90) | 13 (81.25) | 28 (60.87) | 0.122 |
| 2 | n (%) | 25 (51.02) | 3 (18.75) | 14 (30.43) | ||
| 3 | n (%) | 2 (4.08) | 0 (0.00) | 3 (6.52) | ||
| 4 | n (%) | 0 (0.00) | 0 (0.00) | 1 (2.17) | ||
| Pre-pregnancy BMI | kg/m2 | Me (25–75%) | 24.21 (21.51–26.70) | 21.62 (19.47–25.06) | 22.75 (20.70–24.91) | 0.092 |
| Min.–max. | 16.41–32.95 | 17.01–35.16 | 17.36–30.82 | |||
| Pregnancy BMI | kg/m2 | Me (25–75%) | 29.14 (27.28–30.80) | 27.42 (25.71–31.71) | 28.26 (25.82–30.45) | 0.297 |
| Min.–max. | 21.63–38.97 | 22.55–42.19 | 20.83–34.93 | |||
| Weight gain during pregnancy | kg | Me (25–75%) | 14 (11–18) | 17 (14–19) | 14 (11–17) | 0.494 |
| Min.–max. | 2–24 | 2–25 | 6–24 | |||
| % | Me (25–75%) | 21.67 (15.15–27.59) | 30.20 (19.09–33.91) | 22.02 (16.92–28.57) | 0.203 | |
| Min.–max. | 3.08–40.00 | 2.63–36.36 | 8.96–42.86 | |||
| Newborn weight | kg | Me (25–75%) | 3.50 (3.32–3.85) | 3.62 (3.41–3.80) | 3.51 (3.12–3.85) | 0.476 |
| Min.–max. | 2.50–4.60 | 3.18–4.03 | 2.66–4.30 | |||
| Newborn height | cm | Me (25–75%) | 54 (53–57) | 56 (53–57) | 54 (53–56) | 0.918 |
| Min.–max. | 48–62 | 50–58 | 50–60 | |||
| Newborn gender | Male | n (%) | 21 (42.86) | 10 (62.50) | 20 (43.48) | 0.356 |
| Female | n (%) | 28 (57.14) | 6 (37.50) | 26 (56.52) | ||
| Apgar score at 1 min | 7 | n (%) | 0 (0.00) | 0 (0.00) | 1 (2.17) | 0.065 |
| 8 | n (%) | 6 (12.24) | 1 (6.25) | 0 (0.00) | ||
| 9 | n (%) | 11 (22.45) | 2 (12.50) | 4 (8.70) | ||
| 10 | n (%) | 32 (65.31) | 13 (81.25) | 41 (89.13) | ||
| Apgar score at 5 min | 7 | n (%) | 0 (0.00) | 0 (0.00) | 1 (2.17) | 0.188 |
| 8 | n (%) | 1 (2.04) | 0 (0.00) | 0 (0.00) | ||
| 9 | n (%) | 8 (16.33) | 0 (0.00) | 2 (4.35) | ||
| 10 | n (%) | 40 (81.63) | 16 (100.00) | 43 (93.48) | ||
| Duration of uterine contractions | h | Me (25–75%) | – | – | 5.46 (3.50–7.67) | – |
| Min.–max. | – | – | 1.17–12.12 | |||
| Analgesia* | Yes | n (%) | 49 (100.00) | 16 (100.00) | 31 (67.39) | – |
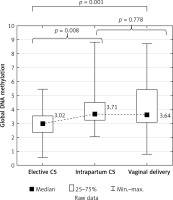
The uterine contraction lasted from 1 to 12 h in vaginal deliveries (5.5 h on average). Spinal analgesia was administered in all the women delivering by caesarean sections, and epidural analgesia in 67% of the vaginal deliveries. In the elective caesarean sections, global DNA methylation in the placenta was significantly lower (3.02 on average) compared to the intrapartum caesarean sections (3.71 on average, p = 0.008), and in relation to the vaginal deliveries (3.64 on average, p = 0.001).
Placental global DNA methylation did not significantly differ between the intrapartum caesarean sections and the vaginal deliveries (p = 0.778; Figure 1).
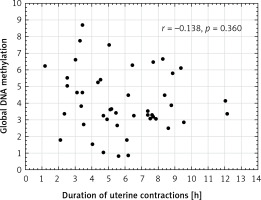
No correlation was found between global DNA methylation in the placenta and the duration of uterine contractions in the vaginal deliveries (r = –0.138, p = 0.360; Figure 2). Placental global DNA methylation did not correlate with the maternal and gestational age, parity, pre-pregnancy and pregnancy BMI, weight gain during pregnancy, newborn weight and height, or with the Apgar score both at 1 and 5 min in all mode of birth groups (Table II).
Table II
Correlations between placental global DNA methylation and maternal and offspring outcomes
| Characteristics | IU | Test* | Elective caesarean section (N = 49) | Intrapartum caesarean section (N = 16) | Vaginal delivery (N = 46) | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | Estimate | P-value | |||
| Maternal age | Years | r | 0.099 | 0.498 | 0.204 | 0.449 | –0.055 | 0.716 |
| Gestational age | Weeks | Z | 1.880 | 0.060 | 0.680 | 0.497 | 1.622 | 0.105 |
| Parity | Number | r | 0.064 | 0.664 | –0.122 | 0.654 | 0.064 | 0.671 |
| Pre-pregnancy BMI | kg/m2 | r | –0.048 | 0.744 | –0.358 | 0.174 | –0.161 | 0.284 |
| Pregnancy BMI | kg/m2 | r | –0.113 | 0.438 | –0.469 | 0.067 | –0.274 | 0.066 |
| Weight gain during pregnancy | kg | r | 0.029 | 0.844 | –0.403 | 0.121 | –0.210 | 0.161 |
| % | r | 0.004 | 0.979 | –0.099 | 0.716 | –0.096 | 0.526 | |
| Newborn weight | kg | r | –0.044 | 0.764 | –0.490 | 0.054 | –0.037 | 0.805 |
| Newborn height | cm | r | –0.190 | 0.191 | –0.042 | 0.877 | 0.231 | 0.122 |
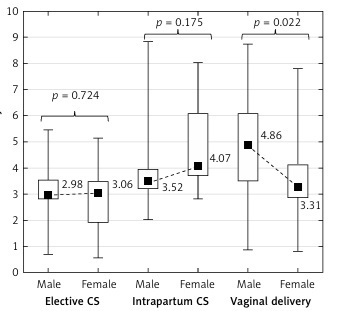
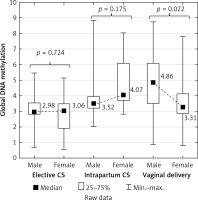
| Newborn gender | Male vs. female | Z | 0.354 | 0.724 | 1.356 | 0.175 | 2.294 | 0.022 |
| Apgar at 1 min | Score | r | 0.014 | 0.923 | –0.357 | 0.175 | 0.046 | 0.760 |
| Apgar at 5 min | Score | r | –0.072 | 0.624 | # | 0.032 | 0.831 | |
| Analgesia | Epidural vs. none | Z | – | – | – | – | 1.640 | 0.101 |
Figure 2
Scatter plot between global DNA methylation in placenta and duration of uterine contractions in vaginal deliveries (n = 46)
Global DNA methylation in the placenta in the male newborns was significantly higher (4.86 on average) than in the female newborns (3.31 on average) in the vaginal deliveries (p = 0.022) but not in either the elective or the intrapartum caesarean sections (p = 0.724 and p = 0.175, respectively) (Figure 3).
Placental global DNA methylation did not significantly differ between women with spinal analgesia and women with epidural analgesia (p = 0.154).
Discussion
Placental tissue is not homogenous in terms of methylation values. Although it globally shows hypomethylation, there are some domains of low methylation (large partially methylated domains (PMDs)), which are surrounded by domains of high methylation (highly methylated domains (HMDs)). Interestingly, in PMDs there are smaller islands characterised by hypermethylation of promoter CpG, but the global methylation for this domain still shows aggregate hypomethylation [26]. Genes in PMDs are mostly silenced, which guarantees the specific function of a given tissue. Similar hypomethylation islands are visible in neoplasms [27], for example in breast cancer [13]. Many environmental and lifestyle factors have now been linked to a disrupted DNA methylation profile in the placenta. Smoking [20, 21], alcohol consumption [28], plastic exposure [29], maternal chronic stress [22], maternal diabetes [23], and maternal pre-pregnancy obesity [30], for example, have been shown to affect both global and gene-specific placental methylation.
The placenta is a uniquely suited organ for the study of how genetics (foetal cells) and environment (e.g. maternal health and nutrient provision) interact, and it may provide considerable insights into the developmental programming of disease. The differences in DNA methylation between individuals born by caesarean section and by vaginal delivery represent an interesting hypothesis yet to be fully investigated. A select panel of different methylated gens via caesarean section could be used as a potential test to diagnose placental disorders or to predict foetal outcomes.
In our study, the global DNA methylation levels in the placenta in the elective caesarean sections were significantly lower compared to the vaginal delivery group (p = 0.001). However, the placental global DNA methylation levels did not significantly differ between the intrapartum caesarean sections and the vaginal deliveries (p = 0.778). Viriani et al. also observed a significantly lower methylation in elective caesarean sections but not in emergency caesarean sections, compared with vaginal deliveries [31]. Franz et al. detected global DNA methylation in umbilical cord blood and did not observe a significant difference in global DNA methylation depending on the mode of delivery, but they showed significantly higher methylation of single genes in newborn infants delivered by elective caesarean section [32]. Some studies have shown that stem cells from infants delivered by caesarean section were globally more methylated than DNA from infants delivered vaginally [33]. The different methods used in these studies have made it difficult to interpret the results of global methylation.
Our study shows significantly higher global DNA methylation in the intrapartum caesarean section group compared to the emergency caesarean section group (p = 0.008). This means that uterine constrictions increase placental global DNA methylation; however, the duration of uterine contractions did not correlate with global methylation (r = –0.138, p = 0.360). It may be concluded from the obtained results that the stress associated with delivery may be necessary to complete the methylation process occurring in the placenta during pregnancy, and a caesarean section eliminates this key part. It also may cause both hypo- and hypermethylation of loci of specific genes and explain the hypothesis of the long-term impact of a caesarean section.
This study represents a pilot study, and hence one limitation is that the number of intrapartum caesarean sections is relatively small (16 participants); nevertheless, the biological significance of the observed phenomenon remains an open question.
DNA methylation is an important regulator of gene function. Foetal sex determination is associated with the risk of several specific pregnancy complications related to placental function. Male foetuses have more severe placental histopathological lesions [34] and a higher placental production of endotoxin-induced tumour necrosis factor response than female foetuses [35]. However, the association between foetal sex and placental DNA methylation remains poorly understood. We emphasise the contrast between global DNA methylation and newborn genders. Placental global DNA methylation was significantly higher (4.86 on average) in the male newborns than in the female newborns (3.31 on average) in vaginal deliveries (p = 0.022), but not in either the elective or the intrapartum caesarean sections (p = 0.724 and p = 0.175). Further investigations will be continued to explain this phenomenon. Martin et al. [36] demonstrated sexual dimorphism at the level of the human placental DNA methylation and hypothesised that epigenetic dimorphism could result in the sex-dependent transport of toxicants, nutrients, and signalling molecules across the placenta. These differences could have consequences for both early- and later-life health outcomes, e.g. hypertension has to been diagnosed more often among men but not women [16].
Many studies have investigated the maternal impact on molecular and functional changes between male and female foetuses. In pre-eclampsia, significant changes were found only in placentas from female foetuses [37]. Sex differences were observed in DNA methylation associated with the summative maternal socioeconomic status score, in which placentas derived from female pregnancies showed more robust differential CpG methylation than placentas from male pregnancies. Moreover, maternal socioeconomic status adversity was associated with the differential methylation of genes with a key role in gene transcription and placental function, potentially altering immunity and stress response [38], but none of the studies have investigated uterine constriction and sex differences in DNA methylation.
In conclusion, a caesarean section performed without uterine contractions significantly influences global DNA methylation in the placenta. In the obtained results, methylation is lower than in vaginal deliveries, and the significance of the differences is not impaired by other factors. Furthermore, this study demonstrates sexual dimorphism at the level of placental global DNA methylation. Further studies investigating different panels of genes may help to identify genes with aberrant methylation in newborn infant delivery by caesarean section compared to vaginal delivery, and could help to demonstrate sexual dimorphism.