Introduction
Atrial fibrillation (AF) is the most commonly encountered sustained cardiac arrhythmia, and its prevalence increases sharply after 65 years of age [1]. Over 10% of individuals aged 80 years or more have AF [2]. Recent projections based on the Rotterdam study suggest that from 2015 to 2050, the number of adults aged > 75 years with AF in the European Union will more than double, rising from 6.3 to 12.9 million [3]. The aetiology, course of illness, and thromboembolic outcomes in older patients with AF might differ from those in the younger population with AF [4]. The pathogenesis of AF in the young with structurally normal atria but electrophysiological “triggers” in the form of pulmonary vein ectopic foci is in stark contrast to that observed in older patients, who develop AF primarily due to structural abnormalities including atrial fibrosis and dilatation as well as systemic disorders such as chronic kidney disease (CKD) [5, 6]. Haemodynamic consequences of loss of atrial contraction in AF are usually more pronounced in older patients with stiffer left ventricles compared to young individuals [1]. Several structural and functional cardiac abnormalities are associated with significant left atrial appendage (LAA) stasis, which may lead to LAA thrombus formation [7]. However, it is not known whether age itself is truly associated with an increased tendency for LAA thrombi formation. Whereas some studies showed that age itself does not predict the presence of LA thrombus [8], some indicated an increased risk of LA thrombus formation with older age [9]. However, all current data are based on small numbers of patients.
In the study reported herein, we aimed to compare LAA thrombus prevalence and its predictors between old and young patients with AF.
Material and methods
Study population
This observational study, conducted in 3 high-reference cardiology departments (in an academic, military, and district hospital), included consecutive patients with AF who underwent transoesophageal echocardiography (TEE) before AF direct current cardioversion or ablation (pulmonary vein isolation) between 2014 and 2018.
The patients were divided into 2 age groups (< 65 and ≥ 65 years). Then, the older group was further divided into 2 groups for an additional subanalysis (65–74 and ≥ 75 years).
Procedures and data collection
Data (including laboratory tests and echocardiography results) were gathered retrospectively from medical records. Patients were included in the study regardless of the presence or type of anticoagulant treatment prior to TEE.
In the academic department, all patients have TEE performed routinely before direct current cardioversion of AF or catheter ablation for AF (pulmonary vein isolation), irrespective of the presence or type of anticoagulation. The only exception are patients admitted for emergency indications [10]. In the district and military hospitals, TEE preceding AF cardioversion or ablation was performed in case of any doubt regarding the efficacy of anticoagulant treatment or patient compliance. In all departments, TEE was conducted within 48 h prior to the scheduled procedure (usually directly or a few hours before the procedure). All TEE studies were performed by certified echocardiographers (certified with accreditation of the Section of Echocardiography of the Polish Cardiac Society), using an EPIQ 7 Ultrasound Machine® (Philips Medical Systems, Andover, Massachusetts, United States), iE33 Ultrasound Machine® (Philips Medical Systems), General Electric Vivid 7 (GE Healthcare, Milwaukee, Wisconsin, United States), or E95 Ultrasound Machine® (GE Healthcare). In cases of left atrial (LA) thrombus suspicion, the study was evaluated by a second echocardiographer, and if any doubt persisted, by a third echocardiographer, to establish the most reliable and unanimous diagnosis, and to enable safe referral for cardioversion or ablation. Written informed consent for TEE was obtained from all patients. In patients with LAA thrombus, ablation or cardioversion was postponed and an intensified anticoagulant regimen was initiated.
The study protocol was submitted to the Ethics Committee, which approved the research protocol and retrospective review of medical records. The Committee waived the requirement to obtain informed consent from the patients.
Definitions
Chronic kidney disease was defined as abnormalities of kidney structure or function (any of the following: albuminuria (albumin excretion rate ≥ 30 mg/day or albumin-to-creatinine ratio ≥ 30 mg/g), urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging, history of kidney transplantation, glomerular filtration rate (GFR) < 60 ml/min/1.73 m2) present for 3 or more months, with implications for health [11].
Heart failure (HF) was defined as a clinical syndrome characterised by typical symptoms (e.g. breathlessness, ankle swelling, fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles, peripheral oedema) caused by structural and/or functional cardiac abnormality, resulting in reduced cardiac output and/or elevated intracardiac pressure at rest or during stress [12].
Statistical analysis
Data were presented as median and interquartile range, mean + standard deviation, or number of patients and percentages, where appropriate. Differences in medians were compared using the Kruskal-Wallis test, and differences in means were compared using Student’s t-test. Frequencies of parameters or events were compared using χ2 test or Fisher’s exact test, as appropriate. For all tests, a p-value < 0.05 was considered to be statistically significant. To determine predictors of LA thrombus on TEE in both age groups, univariate and multivariate logistic regression analyses were performed. Supplementary Table SI presents variables included in univariate logistic regression analyses. Only variables that were available for more than 92% of patients were included in the logistic regression analysis. The multiple logistic regression model included all variables found to be predictors of LA thrombus in univariate analyses. Statistical analysis was performed with SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 1970 patients with AF were included in the study. Patients were divided into 2 groups: 1148 (58.3%) patients aged < 65 years and 822 patients (41.7%) aged ≥ 65 years, including 182 patients (22.1% of the 822 patients) aged ≥ 75 years. Compared to the younger group, patients aged ≥ 65 were twice more often women, and, as expected, were burdened with more concomitant diseases. Detailed clinical characteristics of both age groups are shown in Table I.
Table I
Clinical characteristics, thromboembolic, and bleeding risk in patients aged less than 65 compared to patients aged 65 years and older
Oral anticoagulation (OAC) was prescribed in 799 (97.2%) patients aged ≥ 65 years compared to 1054 (91.8%) in those aged < 65 years (p < 0.001). Among patients treated with OAC, those aged ≥ 65 years less often received vitamin K antagonist (VKA) (267 (33.4%) vs. 416 (39.5%)) and more often non-VKA-OAC (NOAC) (532 (66.6%) vs. 638 (60.5%), p = 0.008) compared to patients younger than 65 years. In patients receiving NOAC, patients aged ≥ 65 years were more often prescribed reduced doses of NOAC compared to the younger group (79 (14.8%) vs. 19 (3.0%), p < 0.001). No significant differences in relation to antiplatelet therapy were seen between the 2 groups. Details on antithrombotic treatment in both age groups are presented in Supplementary Table SII.
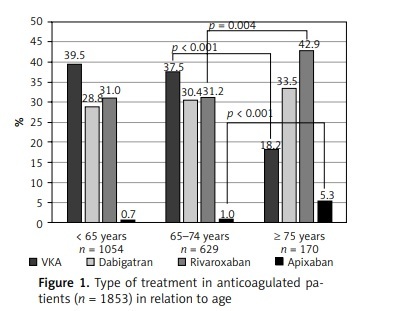
While comparing the 2 prespecified subgroups of older patients, those aged ≥ 75 years were less often prescribed OAC than patients aged 65–74 years (Supplementary Table SII). As shown in Figure 1, in anticoagulated patients, the frequency of VKA therapy decreased and the frequency of NOAC therapy (in particular with rivaroxaban and apixaban) increased after the age of 75 years. Reduced doses of NOAC were used in 53 (38.1%) patients aged ≥ 75 years, and in 26 (6.6%) of those aged 65–74 years (p < 0.001), as shown in Supplementary Table SII. The appropriate reduced doses were prescribed to 24 (68.6%), 23 (57.5%), and 4 (100%) patients in the dabigatran, rivaroxaban, and apixaban groups, respectively (Supplementary Table SIII).
Figure 1
Type of treatment in anticoagulated patients (n = 1853) in relation to age
VKA – vitamin K antagonist. For pairwise comparisons between groups (< 65 years vs. 65–74 years, and 65–74 years vs. ≥ 75 years), only p-values of < 0.05 are given.
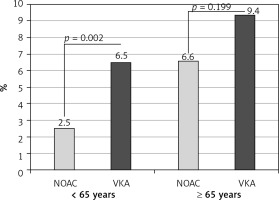
On TEE, LA thrombi were detected in 109 (5.5%) patients. All of those thrombi were found in LAA. The frequency of LAA thrombus was higher in patients ≥ 65 years compared to those < 65 years (63 (7.7%) vs. 46 (4.0%), p < 0.001; as shown in Table II). There was an absolute, although statistically insignificant, difference in LAA thrombus prevalence between patients aged 65–74 years and ≥ 75 years (47 (7.3%) vs. 16 (8.8%), p = 0.528; as shown in Supplementary Table SIV). In patients aged ≥ 65 years, there was no statistically significant difference in the frequency of LAA thrombus between patients treated with VKA and NOAC, in contrast to patients aged < 65 years, in whom NOAC treatment was associated with significantly lower LAA thrombus prevalence, as presented in Table III and Figure 2.
Table II
Laboratory and echocardiographic characteristics of patients aged less than 65 years compared to patients aged 65 years and older
| Variable | Patients aged < 65 years (n = 1148) | Patients aged ≥ 65 years (n = 822) | P-value |
|---|---|---|---|
| Laboratory parameters: | |||
| Haemoglobin (g/dl] | 15 (14–16) n = 1132 | 14 (13–15) n = 801 | < 0.01 |
| Haematocrit (%) | 43 (41–46) n = 999 | 41 (39–44) n = 643 | < 0.01 |
| WBC (K/µl] | 7.4 (6.3–8.8) n = 1058 | 7.3 (6.1–8.5) n = 682 | 0.03 |
| Platelet count (K/µl] | 219 (185–252) n = 1132 | 210 (174–252) n = 795 | 0.01 |
| GFR (ml/min/1.73 m2] | 79 (65–90) n = 1035 | 65 (53–89) n = 818 | < 0.01 |
| AST (U/l] | 24 (20–30) n = 865 | 24 (20–29) n = 553 | 0.21 |
| ALT (U/l] | 32 (24–43) n = 873 | 25 (19–35) n = 554 | < 0.01 |
| INR (in patients on VKA) | 1.2 (1.0–2.1) n = 384 | 1.4 (1.1–2.2) n = 235 | < 0.01 |
| Transthoracic echocardiography*: | |||
| Ejection fraction (%) | 57 (45–60) n = 418 | 57 (50–60) n = 409 | 0.96 |
| Left atrial diameter (mm] | 45 (41–49) n = 512 | 45 (41–48) n = 376 | 0.19 |
| Left ventricular diastolic diameter (mm] | 53 (49–57) n = 302 | 52 (47–57) n = 296 | 0.08 |
| Transoesophageal echocardiography*: | |||
| Thrombus (n (%)] | 46 (4.0%) | 63 (7.7%) | < 0.01 |
| LAA emptying velocity (cm/s] | 52 (34–76) n = 925 | 40 (27–60) n = 556 | < 0.01 |
| SEC (n (%)] | 187 (19%) | 154 (24%) | 0.17 |
Table III
Comparison of patients on different anticoagulant regimens in relation to age
| Variable | Patients aged < 65 years | Patients aged ≥ 65 years | ||||
|---|---|---|---|---|---|---|
| VKA (n = 416) | NOAC (n = 638) | P-value | VKA (n = 267) | NOAC (n = 532) | P-value | |
| Age [years) | 58 (52–61) | 58 (52–64) | 0.59 | 69 (67–72) | 70 (67–75) | < 0.01 |
| Female [n (%)) | 100 (24%) | 170 (27%) | 0.35 | 138 (52%) | 274 (52%) | 1.00 |
| Paroxysmal AF [n (%)) | 236 (57%) | 298 (47%) | < 0.01 | 146 (55%) | 169 (32%) | < 0.01 |
| Non-paroxysmal AF [n (%)) | 180 (43%) | 340 (53%) | < 0.01 | 121 (45%) | 363 (68%) | < 0.01 |
| Previous ischaemic stroke/TIA/peripheral embolism [n (%)) | 17 (4.1%) | 37 (5.8%) | 0.25 | 31 (12%) | 49 (9.2%) | 0.32 |
| Previous bleeding [n (%)) | 13 (3.1%) | 24 (3.8%) | 0.61 | 11 (4.1%) | 45 (8.5%) | 0.03 |
| Thromboembolic and bleeding risk: | ||||||
| CHADS2 score | 1 (1–2) | 1 (0–1) | 0.08 | 1 (1–2) | 2 (1–3) | 0.09 |
| CHA2DS2-VASc score | 1 (1–2) | 1 (1–2) | 0.15 | 3 (2.5–4) | 3 (3–5) | 0.28 |
| HAS-BLED score | 1 (0–1) n = 337 | 1 (0–1) n = 465 | 0.31 | 2 (2–2) n = 187 | 2 (2–3) n = 359 | 0.31 |
| Transoesophageal echocardiography*: | ||||||
| Thrombus [n (%)) | 27 (6.5%) | 16 (2.5%) | < 0.01 | 25 (9.4%) | 35 (6.6%) | 0.20 |
| LAA emptying velocity [cm/s) | 54 (32–75) n = 367 | 50 (33–72) n = 467 | 0.64 | 47 (30–67) n = 224 | 36 (25–56) n = 310 | < 0.01 |
| SEC [n (%)) | 81 (21%) n = 387 | 100 (19%) n = 525 | 0.11 | 52 (22%) n = 235 | 99 (26%) n = 386 | 0.77 |
Figure 2
Prevalence of left atrial appendage thrombus in relation to age and type of anticoagulation
NOAC – non-vitamin K antagonist oral anticoagulants, VKA – vitamin K antagonists.
In multivariate logistic regression, predictors of LAA thrombus in patients aged ≥ 65 years were: older age, non-paroxysmal AF (vs. paroxysmal AF), HF, and lower GFR and platelet count (as shown in Table IV), whereas in patients aged < 65 years – older age, non-paroxysmal AF (vs. paroxysmal AF), HF, and VKA use (compared to NOAC therapy), as shown in Table V. In patients aged ≥ 65 years, no type of OAC treatment (VKA vs. NOAC; reduced doses of NOAC) predicted LAA thrombus.
Table IV
Logistic regression analyses of predictors of left atrial thrombus in the group of patients aged 65 years or older
Table V
Logistic regression analyses of predictors of left atrial thrombus in patients aged less than 65 years
Despite the fact that HF appeared to be a significant predictor of LAA thrombus in both age groups, no statistically significant differences were observed between patients aged < 65 years and those aged ≥ 65 years according to ejection fraction (57 (45–60) vs. 57 (50–60), p = 0.96), LA diameter (45 (41–49) vs. 45 (41–48), p = 0.19), and left ventricular diastolic dimension (53 (49–57) vs. 52 (47–57), p = 0.08) (Table II) as well as between patients those aged 65–74 and ≥ 75 – ejection fraction (58 (50–60) vs. 55 (50–60), p = 0.19), LA diameter (45 (42–48) vs. 46 (42–51), p = 0.20), and left ventricular diastolic dimension (52 (47–57) vs. 50 (46–57), p = 0.65) (Supplementary Table SIV).
Discussion
The major findings of the present study are as follows. First, despite OAC use in most, older patients with AF remain at high risk of LAA thrombus formation. Second, in both age groups, older age, non-paroxysmal AF (vs. paroxysmal AF), and HF proved to be strong predictors of LAA thrombus. Third, in the older patients, age and GFR were related to the risk of thrombus formation. Last, in patients aged < 65 years, NOAC treatment was associated with a lower risk of LAA thrombus compared to VKA therapy.
The attributable risk of thromboembolic events increases sharply with age, from 1.5% for patients aged 50 to 59 years to 23.5% for patients aged 80 to 89 years, and with 40% of stroke in those > 80 years due to AF [13]. In a sub-analysis of the PREFER in AF (PREvention of Thromboembolic Events-European Registry in Atrial Fibrillation) study, the prevalence of thromboembolic events was 4.3% per year in AF patients over 85 years old and 2.3% per year in patients with AF who were younger than 85 years, despite antithrombotic treatment [14]. In a meta-analysis including 8932 patients from 12 randomised trials on stroke prevention in AF, the risk of ischaemic stroke increased by age, with a 45% increase in risk for every 10 years starting at the age of 50 years [15].
Historically, the risk of thromboembolism has been considered to be independent of the AF pattern [16–19]. This consensus of risk equivalence between AF types is reflected in current North American [17] and European AF guidelines [16]. However, recent reports suggest that the pattern of AF is a strong predictor of stroke, and division of AF into paroxysmal and non-paroxysmal AF is gaining widespread acceptance in clinical practice. In the study by Vanassche et al., the annual rate of thromboembolism increased from 2.1% in paroxysmal AF to 3.0% in persistent and 4.2% in permanent AF, resulting in a hazard ratio for non-paroxysmal vs. paroxysmal of 1.91 (95% confidence interval (CI) 1.50–2.43; p < 0.001) [20]. A recent meta-analysis by Ganesan et al., including almost 100,000 patients, reported a 38% greater risk of thromboembolism and a 22% increase in mortality in non-paroxysmal AF as compared to paroxysmal AF [21]. Whether AF type is indeed an independent risk factor for stroke or rather a reflection of patients’ thromboembolic risk profile is yet to be determined.
HF is a well-recognised predictor of stroke in patients with AF [22–25]. In our study, HF was associated with more than doubled risk of LAA thrombus in both age groups. Similarly to AF, HF is a growing epidemic and its prevalence increases with age [26]. When present in combination, AF and HF portend a worse prognosis than either condition alone, with a 4-fold increased risk of systemic thromboembolism events per year [27]. Moreover, HF is a negative predictor of LAA thrombus resolution in patients with AF receiving OAC [23].
Another factor related to increased risk of LAA thrombus in our study was lower GFR. Patients with advanced kidney disease are known to be at increased bleeding risk (Piccini et al., 2013; Singer et al., 2013). In both the AnTicoagulation and Risk factors In AF (ATRIA) study [28] and the R2CHADS2 score [29], renal dysfunction defined as GFR < 45 ml/min/1.73 m2 and < 60 ml/min/1.73 m2, respectively, was related to higher thromboembolic risk and enhanced its stratification compared to the CHA2DS2-VASc and CHADS2 scores. Recently, we proposed a new cut-off of 56 ml/min/1.73 m2 for an improved thrombus risk stratification in AF [22]. The lack of association between GFR and the presence of LAA thrombus in patients younger than 65 years in our study was probably due to the low prevalence of renal dysfunction in these patients.
In our study, the older the patient, the higher the risk for LAA thrombus formation (a known observation reflected in the CHA2DS2-VASc score), and this rule applies even among patients younger than 65 years. In the Framingham Study, the percentage of stroke attributable to AF increased steeply, from 1.5% at 50–59 years of age, through 2.8% at age 60–69 years and 9.9% at 70–79 years, to 23.5% at 80–89 years of age [30]. The findings of the above and further studies showed that octogenarian would be burdened with a substantially higher thromboembolic risk than for a 70-year-old patient [13, 14]. In our study, the prevalence of LAA thrombus in patients aged 75 years and older was higher than in patients aged 65–74 years (8.8% vs. 7.3%), although the difference was not statistically significant, which might have been related to a low number of patients ≥ 75 years.
Among physicians, the most commonly reported concern with anticoagulation is the fear of falls and consequent bleeding [31]. This has resulted in OAC underuse, especially in older patients [32, 33]. However, more recently, there has been a progressive increase in the proportion of older patients with AF receiving guideline-recommended therapy. In the Prevention of Thromboembolic Events-European Registry in AF 65 years) [34]. In the Global Anticoagulant Registry in the FIELD-AF (GARFIELD-AF), OAC use increased from 55 to 74% during 5-year observation from 2011 to 2016. Of particular relevance to older patients, the mean age in this study was 75 years, with 87% of patients being aged 65 years or older [35]. This positive trend is also reflected in our study, with 97.2% of patients aged ≥ 65 years receiving OAC. Still, there were significant differences in the frequency of OAC use between patients from different age groups. While lower frequency of OAC use in patients < 65 years of age (91.8%) as compared to patients aged 65–74 years (98.3%) may be explained by their lower CHA2DS2-VASc score (with a median score of 1 point, which is a class IIa indication to OAC), lower frequency of OAC use in patients aged ≥ 75 years (93.4%) suggests therapeutic inertia, given that there was no difference in the prevalence of history of bleeding between the 2 older groups.
In landmark clinical trials, NOACs have consistently demonstrated to be associated with lower rates of intracranial haemorrhage compared with VKA [36–39]. In ARISTOTLE, apixaban was also reported to cause major bleeding less often than warfarin [40]. These aspects may explain the preference for the prescription of NOAC among patients aged ≥ 65 years in our study, especially apixaban among the oldest age group (> 75 years).
There is also growing evidence of a possible reduction of thromboembolic risk of NOAC compared to VKA [38, 41, 42]. Recent meta-analyses demonstrated similar or improved efficacy in thromboembolism prevention for NOAC compared to VKA [43–45]. This also reflected in older patients with AF [44, 45]. In our study, NOAC treatment was shown to be associated with lower risk of LAA thrombus as compared to VKA therapy in patients younger than 65 years. Notably, one third of patients on VKA with known international normalised ratio were under-anticoagulated, with an international normalised ratio below 2. In the older (≥ 65 years) group, LAA thrombus prevalence was also lower in patients receiving NOAC (6.6%) compared to VKA-treated patients (9.4%), although the difference did not reach statistical significance, possibly due to the smaller sample size but perhaps also due to substantially increased thrombotic risk in the older group.
Our study has several limitations. Firstly, because this study was based on retrospective data collection, some variables were not available for all the patients, as indicated in the tables. Secondly, inclusion of patients with AF routinely referred for TEE enabled assessment of the presence of LAA thrombus but limited the study group to patients scheduled for cardioversion or ablation, who are younger and at lower thromboembolic risk than the population with AF in general; hence, there is a high risk that patients with chronic AF might not be included. Therefore, there should be caution in generalising our findings to all patients with AF, because results may differ in other patient populations. Thirdly, the limitation of our study is also the fact that our study included only patients with AF who were qualified for ablation or cardioversion. This means that our study did not include a large group of elderly patients with AF who simply were not qualified for ablation or cardioversion, because they were too old and/or had a higher comorbidity burden. So, in fact, the results of this analysis cannot be simply extrapolated to the entire population of older patients with AF. Fourthly, all the results may be biased due to their observational nature. Finally, this was not a randomised controlled study comparing NOAC with VKA, and thus no ultimate conclusion on the relative efficacy of either OAC regimen can be drawn from our analysis.
In conclusion, despite the use of OAC, older patients with AF remain at high risk of LAA thrombus formation. Age, and non-paroxysmal AF and HF are predictors of LAA thrombus, irrespective of age. In the older patients, impaired kidney function and older age are also associated with higher presence of LAA thrombi.