The sodium-glucose cotransporter 2 inhibitors (SGLT2i) or gliflozins are considered as a major class in the therapeutic strategy of type 2 diabetes mellitus (T2DM) due to their proven efficacy in reducing blood sugar levels and, more importantly, in decreasing major cardiovascular events (MACE) [1].
This new class of drugs has sparked tremendous enthusiasm among the medical community, due to its impressive and unexpected reduction of MACE in heart failure (HF) patients and chronic kidney disease (CKD) patients, diabetic as well as non-diabetic [2, 3]. Thus, SGLT2i have been compared to paradigm-shifting drugs such as Aspirin and penicillin for their major clinical benefits, leading to the question recently posed: “Could flozins be the statins for risk-based primary prevention of heart failure?” [4, 5]. Hence, the question arises spontaneously: should a SGLT2i be prescribed in all T2DM patients?
SGLT2i act on the proximal tubule of kidney and inhibit renal glucose’s reabsorption, increasing glucose and sodium excretion, with an excellent safety profile [6].
In 2015, the first cornerstone randomized controlled trial (RCT) was published: the Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG) Trial [2]. In this placebo-controlled trial, 7,020 patients with type 2 diabetes and established cardiovascular disease were randomized to receive either empagliflozin or placebo. Compared to the placebo arm, the SGLT2i arm surprisingly had a lower risk of death from cardiovascular causes (HR = 0.62; 95% CI: 0.49 to 0.77; p < 0.001), hospitalization for heart failure (HHF) (HR = 0.65; 95% CI: 0.50 to 0.85; p = 0.002), and death from any cause (HR = 0.68; 95% CI: 0.57 to 0.82, p < 0.001).
The next randomized study evaluated the impact of dapagliflozin in the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction (DECLARE-TIMI 58) trial [7]. In this RCT, T2DM patients at risk for atherosclerotic cardiovascular disease or in secondary prevention were randomly assigned to dapagliflozin or placebo. A lower rate of cardiovascular death (HR = 0.83; 95% CI: 0.73 to 0.95; p = 0.005) or HHF (HR = 0.73; 95% CI: 0.61 to 0.88) was reported in the dapagliflozin group, confirming its cardiovascular benefits.
Further positive results with SGLT2i were reported in the CANVAS Program, a combination of two RCTs in which 10,142 participants with T2DM and high cardiovascular risk were randomized to receive canagliflozin or placebo [8]. Canagliflozin was found to reduce the risk of cardiovascular events compared to placebo (HR = 0.86; 95% CI: 0.75 to 0.97; p < 0.001 for noninferiority; p = 0.02 for superiority). Notably, the treated arm revealed a greater risk of amputation, not observed in other trials. Another major RCT evaluating canagliflozin versus placebo was the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial [9]. In this study, canagliflozin reduced the risk of kidney failure and cardiovascular events compared to placebo (HR = 0.70; 95% CI: 0.59 to 0.82; p = 0.00001).
In the same therapeutic class, ertugliflozin was evaluated in the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV), which randomized 8,246 patients with type 2 diabetes and atherosclerotic cardiovascular disease to ertugliflozin or placebo [10]. Ertugliflozin was non-inferior to placebo concerning MACE (a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) (HR = 0.97; 95.6% CI: 0.85 to 1.11; p < 0.001), but failed to reduce events.
The high level of proofs of clinical benefits of SGLT2i was reinforced by a meta-analysis (MA) of 6 RCTs in patients with T2DM [11]. In this MA, SGLT2i reduced the risk of MACE (HR = 0.90; 95% CI: 0.85-0.95; Q statistic, p = 0.27), mainly driven by a reduction of HHF and cardiovascular mortality. Nevertheless, apparent heterogeneity was observed: empagliflozin was associated with a lower risk of cardiovascular death, canagliflozin with a lower risk for MACE and incidence of kidney disease, whereas dapagliflozin was associated with a reduced risk for HHF, to be interpreted cautiously due to methodological limitations of this MA.
The spectrum of the clinical benefits of gliflozins has become wider since they are equally effective in diabetic and non-diabetic patients, as reported in heart failure patients and in individuals with a chronic kidney disease (CKD) [12–14]. More recently, the EMPEROR Preserved trial and DELIVER trial reported clinical benefits on hard endpoints in patients with heart failure with preserved ejection fraction (HFpEF), leading to the current perspective of the wide use of SGLT2i among the heart failure population, independently of ejection fraction (EF) [15]. Notably, a recent RCT evaluating dapagliflozin and empagliflozin in HF or CKD versus optimal medical therapy (OMT) reported similar efficacy and safety for both drugs, suggesting a class effect [16].
The underlying mechanisms explaining the cardiovascular and renal benefits of SGLT2i remain not entirely elucidated. Hypotheses include a reduction of preload promoted by osmotic diuresis, a decrease of afterload mediated by improved endothelial function and potential inhibition of cardiac fibrosis [17, 18]. Weight loss resulting from glycosuria, improved endothelial function, modulation of inflammation markers and growth factors may also contribute to the cardiovascular benefits [19]. Due to these cumulative benefits, SGLT2i are indicated in T2DM patients with comorbidities, target organ damage and/or in secondary prevention, often in combination with metformin, which still plays a key role in glycaemic control [20]. Notably, particular concern should be given to patients with latent autoimmune diabetes in adults (LADA), where the assumption of SGLT2i is linked with a higher risk of ketoacidosis [21].
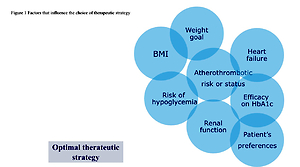
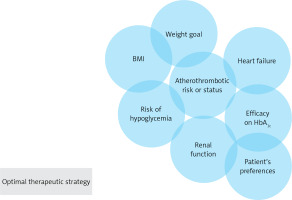
Despite these impressive results, there are also preferred indications for the class of the glucagon-like peptide 1 receptor agonists (GLP1-RA), which reduce MACE in T2DM patients with a more pronounced heterogeneity (Figure 1). Indeed, GLP1-RA provide a more potent decrease of glycated haemoglobin and weight loss in indirect comparison with a good safety profile [22, 23]. In this context, a recent meta-analysis tried to enlighten differences among GLP1-RA and SGLT2i in T2DM patients and assessed their efficacy and safety in this population. The effectiveness of both therapeutic classes was similar when compared with OMT, by reducing MACE with comparable effects on myocardial infarction (MI), cardiovascular mortality, and all-cause mortality [24]. Even though SGLT2i provide better nephroprotection and prevention of HHF than GLP1-RA, some uncertainties remain about their indication in all T2DM patients. Firstly, no RCT of SGLT2i versus OMT reported a reduction in acute coronary syndromes (ACS), whereas the LEADER trial demonstrated the efficacy of liraglutide in this field [25]. Furthermore, GLP1-RA reduced ischemic strokes in the REWIND and SUSTAIN-6 studies, whereas only sotagliflozin in the SOLOIST-WHF trial showed a stroke reduction [26–28].
Interestingly, two ongoing trials, DAPA-MI and EMPACT-MI, recruiting 6,400 and 6,500 patients respectively (in the early phase after an acute MI), are providing additional information on the widespread usage of gliflozins. These RCTs are evaluating the ability of dapagliflozin or empagliflozin to decrease the risk of HHF and cardiovascular death compared with placebo in post-myocardial infarction patients with low left ventricular ejection fraction (LVEF) compared to OMT [29, 30].
Due to the inclusion criteria of these RCTs, questions about the efficacy of gliflozins to reduce ischemic events will remain and require dedicated studies in coronary patients with normal LVEF.
Additionally, evidence suggests that combination therapy with GLP1RA and SGLT2i may improve glycaemic control, weight loss, systolic blood pressure, NAFLD, and renal function. There is therefore a need for further research to refine the combined effects on cardiovascular and renal disease [31, 32].
In conclusion, SGLT2i are a unique story of serendipity [33]. Their wide effects, accidentally discovered, are still changing medical practice and may lower the burden of MACE. Indeed, gliflozins are effective in the following ways: reducing blood sugar, improving outcomes in patients with HFrEF and HFpEF irrespective of T2DM, slowing the development of end-stage kidney disease in patients with chronic kidney disease.
Even though SGLT2i treatment reduces cardiovascular events, its long-term effectiveness is still unknown, particularly in atherosclerosis. In contrast, some GLP1-RA already have proven efficacy in this field, in combination with a more potent decrease in weight and glycated haemoglobin. In this context, the choice of antidiabetic agents in T2DM patients should be tailored to each patient according to all the clinical characteristics in order to apply the most effective strategy. In conclusion, it is crucial to improve the awareness among general practitioners and diabetologists of the high incidence of HF in diabetic patients in order to start SGLT2i therapy earlier.
Conflict of interest
Giuseppe Biondi-Zoccai has consulted for Balmed, Cardionovum, Crannmedical, Eukon, Innovheart, Guidotti, Meditrial, Microport, Opsens Medical, Replycare, Teleflex, and Terumo. Pierre Sabouret reports consulting fees from Astra Zeneca, BI, BMS, Lilly, Les laboratoires Servier, Novartis, Recordati, Sanofi, Vifor outside the submitted work.