Introduction
Neurologic complications are postulated as postoperative side effects after the beginning of on-pump cardiac surgery [1]. Postoperative cognitive dysfunction (POCD) is the most frequently observed complication, with an incidence of 30–79% [2]. It refers to varying degrees of cognitive function decline in patients after surgery, and could affect a variety of domains including memory, information processing, and executive functioning, leading to increased mortality and unexpected complications [3, 4]. The risk of POCD is increased in older adults due to physiologic, pharmacokinetic, and pharmacodynamic changes that are associated with aging [3].
Hepatic ischemia/reperfusion injury (I/R) is a significant source of morbidity and mortality after liver surgery [5]. During the I/R process, the organ suffers ischemia due to a lack of oxygen and nutrients, followed by reperfusion damage induced by free radicals [6–8]. Studies have showed that the nuclear factor erythroid 2-related factor 2 (Nrf2) and nuclear factor κB (NF-κB) are involved in the process of I/R [9, 10]. The hippocampus, a bilateral structure within the middle temporal lobe, is well known for being an essential part of the neural network of learning and memory. During pathological processes such as Alzheimer’s disease, the hippocampal volume decreases more rapidly [11, 12]. Our previous study proved that I/R had a close relationship with the incidence of POCD [13].
The primary aim in this study is to investigate the effect of I/R on hippocampus in rats with POCD.
Material and methods
Animals
Adult male Sprague-Dawley rats (n = 160, age: 20–25 months, weight: 300–350 g) were provided by the Animal Experimental Center of our university. The rats were maintained under temperature-controlled conditions with a 12/12-h light/dark cycle and had ad libitum access to food and water. The study was approved by the medical ethics committee of our university, and in accordance with international ethics guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals.
Rats were randomly divided into 4 groups (40 rats per group): sham group, ischemia for 20 min (I/R [20]) group, ischemia for 30 min (I/R [30]) group, and ischemia for 40 min (I/R [40]) group. A model of segmental (70%) hepatic ischemia was conducted in the study. Rats were fasted for 12 h but allowed free access to water before anesthesia. They were intraperitoneally anaesthetized with sodium pentobarbital (50 mg/kg) and received midline laparotomy. The portal vein and hepatic artery branches were clamped by an atraumatic microvascular clamp. The vascular clamps were removed and reperfusions were allowed after ischemia for 20 min, 30 min, 40 min in different groups. The reperfusion lasted for 30 min.
Anti-infection treatment of intraperitoneal injection with penicillin (72 U/kg/day) was adopted for 3 days after surgery. At the 4th day after surgery, 20 rats received the Morris water maze (MWM) test and the other 20 rats received the passive avoidance test in each group.
Morris water maze test
The Morris water maze test was chosen for evaluating the spatial learning, spatial memory, and cognitive flexibility in the rats [14]. The water maze tank was 160 cm in diameter, 50 cm in depth. A platform submersed 2 cm below the surface was placed in the target quadrant of the tank. Firstly the navigation training session was displayed from the 4th day to the 10th day after surgery. Briefly, rats were released into water facing the wall of the pool from one quadrant. They were allowed to locate the hidden platform and land on it within 90 s [15]. If a rat failed to find the platform within 90 s, it would be guided to the platform and placed on the platform for 10 s. The training session consisted of 4 consecutive trials per day for 7 days. The escape latency to find the platform was recorded every day. Then the platform was removed and the spatial probe test would be conducted from the 11th day to the 18th day after surgery. In this session, rats were released into the water facing the wall of the pool from the contralateral quadrant of the target platform quadrant. The frequency of crossing, swimming speed, path length and time spent in the target quadrant were recorded and calculated. The two sessions included 10 rats each. After each session, the rats in the session would be sacrificed and the hippocampus would be prepared.
Passive avoidance test
The passive avoidance test was conducted from the 4th day to the 18th day after surgery. The apparatus consisted of two compartments, one light (250 × 185 × 300 mm) and the other dark (250 × 185 × 300 mm), connected by a circular hole. The rats were initially placed in the light compartment. After the rat entered the dark compartment, the rat was given a 40 V electric shock. The step-through latency to enter the dark compartment was recorded. When a rat did not enter the dark compartment within 300 s, the step-through latency was recorded as 300 s.
Tissue sampling
After each session of the MWM test and passive avoidance test, the rats were immediately sacrificed by an overdose of sodium pentobarbital (30 mg/kg). The hippocampus was obtained and immersed in artificial cerebrospinal fluid (ACSF). Then hippocampus were proceeded into 40 μm sections and incubated in a thermostatic bath at 34°C for 1.5 h. ACSF with the perfusion speed of 1.5–2 ml/min and a gas mixture (consisting of 95% O2 and 5% CO2) with the flow of 200 ml/min were supplied during the incubation process.
Population spike of pyramidal cells in hippocampus
After incubation for 1.5 h, the sections were placed on a nylon mesh pad of the thermostatic bath. The liquid level was 2 mm higher than the sections. A population spike was evoked from the CA1 pyramidal cell layer following stimulation of the Schaffer collateral fibers in the stratum radiatum near the border of the CA3 region. Recordings were made with a glass microelectrode (impedance of 2–10 MΩ) filled with sodium chloride solution (4 mmol/l) at the CA1 pyramidal cell layer. The electrical stimulation of a single pulse, pulse intensity of 0.2–0.8 mA and pulse width of 0.15 ms was applied. The size of the population spike was measured as the peak-to-peak amplitudes after perfusion for at least 15 min to allow the attainment of stable responses.
Histological analysis
The frozen hippocampus sections were incubated overnight at 4°C with primary antibodies of anti-NF-κB and anti-protein kinase γ (PKCγ). Secondary antibody labeled with fluorescein isothiocyanate (FITC) was prepared for PKCγ. The appropriate secondary antibody without labeled FITC was prepared for NF-κB.
The number of positively stained cells was counted by confocal microscopy at 400× magnification. Nissl staining was performed with Cresyl fast violet.
Statistical analysis
SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA) was employed for statistical analysis. Continuous data were expressed as means ± standard deviation. Continuous variables were statistically analyzed by Student’s t-test. Categorical variables were assessed by χ2 analysis. P < 0.05 was considered as a statistical significance.
Results
Morris water maze test to assess the effect of I/R on spatial learning and memory
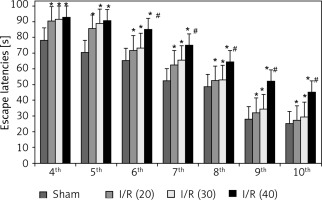
During the navigation training session from the 4th day to the 10th day after surgery, the escape latencies of rats in I/R (20), I/R (30), I/R (40) groups were significant prolonged compared with the sham group (p < 0.05). Rats in the I/R (40) group showed markedly prolonged escape latency compared with the other three groups (p < 0.05) from the 6th day to 10th day after surgery (Figure 1).
Figure 1
Escape latencies of rats during the navigation training session. Compared with sham group, *p < 0.05; compared with sham, hepatic ischemia/reperfusion injury (I/R) (20) and I/R (30) group, # p < 0.05. Data are expressed as mean ± standard deviation
During the spatial probe test at the 11th day after surgery, as shown in Table I, frequency of crossing, swimming speed, path length and time spent in the target quadrant showed significant differences in rats of the I/R (40) group compared with the other three groups (p < 0.05). No significant difference of these parameters was observed between groups at the 18th day after surgery (p > 0.05).
Table I
Spatial probe test at 11th and 18th day after surgery (mean ± SD, n = 20)
| Group | Frequency of crossing | Swimming speed [cm/s] | Path length in target quadrant [cm] | Time spent in target quadrant [s] | ||||
|---|---|---|---|---|---|---|---|---|
| 11th day | 18th day | 11th day | 18th day | 11th day | 18th day | 11th day | 18th day | |
| Sham | 6.18 ±1.25 | 5.72 ±1.14 | 11.25 ±1.34 | 11.63 ±2.46 | 168.65 ±29.52 | 187.63 ±21.18 | 12.89 ±1.63 | 13.81 ±1.62 |
| I/R (20) | 5.91 ±0.92# | 5.83 ±1.14 | 11.31 ±1.10# | 12.25 ±0.92 | 187.21 ±20.45# | 195.30 ±16.61 | 13.15 ±1.26# | 14.41 ±1.57 |
| I/R (30) | 5.81 ±0.87# | 5.72 ±0.63 | 12.14 ±1.32# | 12.95 ±1.12 | 187.11 ±13.16# | 192.78 ±14.34 | 13.12 ±3.07# | 14.12 ±3.24 |
| I/R (40) | 3.21 ±1.95* | 5.76 ±0.13 | 9.50 ±1.42* | 12.18 ±1.03 | 124.34 ±38.21* | 191.32 ±16.46 | 8.79 ±3.18* | 13.53 ±1.28 |
Passive avoidance test to assess the effect of I/R on non-spatial memory
The passive avoidance test was used to analyze non-spatial memory. The I/R (40) group showed marked differences in escape latency and error frequency compared with the other three groups from the 6th day to 8th day after surgery (all p < 0.01) (Tables II and III). It showed no significant difference of escape latency or error frequency between groups from the 11th day to 18th day after surgery (p > 0.05) (Tables IV and V).
Table II
Escape latency from 4th day to 10th day after surgery in passive avoidance test (mean ± SD, n = 40)
| Group | 4th | 5th | 6th | 7th | 8th | 9th | 10th |
|---|---|---|---|---|---|---|---|
| Sham | 134.39 ±7.05 | 167.79 ±6.86 | 201.50 ±3.61 | 258.60 ±1.57 | 289.12 ±1.68 | 293.90 ±1.66 | 298.19 ±12.76 |
| I/R (20) | 62.40 ±3.13*+ | 78.37 ±5.63*+ | 95.49 ±4.72*+ | 169.60 ±8.63*+ | 208.20 ±6.89+ | 263.70 ±5.57+ | 286 ±9.56+ |
| I/R (30) | 52.92 ±2.64*# | 54.32 ±6.98*# | 102.67 ±9.63*+ | 163.82 ±9.69*+ | 197.06 ±15.37+ | 241.40 ±1.24+ | 283 ±9.46+ |
| I/R (40) | 44.12 ±3.56* | 52.23 ±5.37* | 70.25 ±6.44* | 126.39 ±13.89* | 177.31 ±12.42* | 225.66 ±17.26* | 276 ±10.75* |
Table III
Error frequency from 4th day to 10th day after surgery in passive avoidance test (mean ± SD, n = 40)
| Group | 4th | 5th | 6th | 7th | 8th | 9th | 10th |
|---|---|---|---|---|---|---|---|
| Sham | 1.81 ±0.28 | 0.73 ±0.23 | 0.64 ±0.19 | 0.42 ±0.18 | 0.31 ±0.12 | 0.20 ±0.08 | 0.00 ±0.00 |
| I/R (20) | 5.80 ±1.05*+ | 2.62 ±0.63*+ | 1.01 ±0.65+ | 0.60 ±0.41+ | 0.52 ±0.16+ | 0.31 ±0.13+ | 0.00 ±0.00 |
| I/R (30) | 6.79 ±1.64*+ | 4.31 ±0.67*+ | 1.42 ±0.41+ | 0.69 ±0.41+ | 0.40 ±0.12+ | 0.32 ±0.11+ | 0.05 ±0.00 |
| I/R (40) | 8.36 ±1.57* | 5.39 ±1.24* | 2.36 ±0.82* | 2.26 ±0.91* | 1.60 ±0.59* | 1.16 ±0.32* | 0.56 ±0.15+ |
Table IV
Escape latency from 11th day to 18th day after surgery in passive avoidance test (mean ± SD, n = 40)
Table V
Error frequency from 11th day to 18th day after surgery in passive avoidance test (mean ± SD, n = 40)
Expression of PKCγ in CA3 region of hippocampus
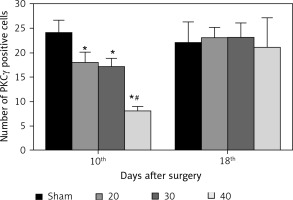
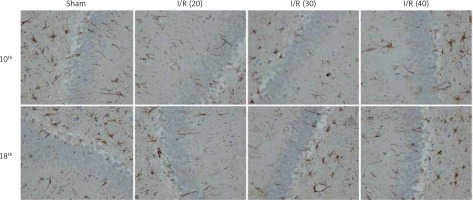
The expression of PKCγ in the CA3 region of the hippocampus was detected. At the 10th day, compared with the sham group, the expression of PKCγ in the I/R (20) group, I/R (30) group and I/R (40) group was significantly lower (p < 0.05). Compared with the other three groups, expression of PKCγ in the I/R (40) group was significantly lower (p < 0.05). No significant difference of the expression of PKCγ was observed between groups at the 18th day after surgery (p > 0.05) (Figures 2 and 3).
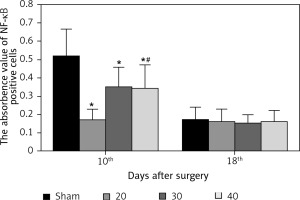
Expression of NF-κB in hippocampus
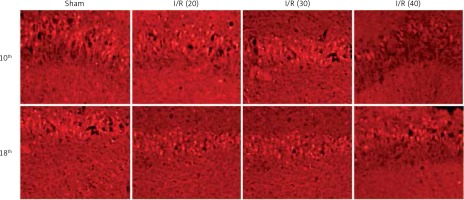
The expression of NF-κB in the CA3 region of the hippocampus was detected. At the 10th day, compared with the sham group, the expression of NF-κB in the I/R (20) group, I/R (30) group and I/R (40) group was significantly lower (p < 0.05). Compared with the other three groups, expression of NF-κB in I/R (40) group lower significantly (p < 0.05). No significant difference of the expression of NF-κB was observed between groups at the 18th day after surgery (p > 0.05) (Figures 4 and 5).
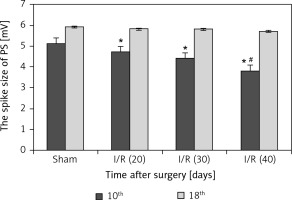
Population spike in hippocampus
Figure 6 reveals that the spike size was markedly reduced in the I/R (40) group compared with the other three groups (p < 0.05), and prolonged peak potential stimulation response time was also observed in the I/R (40) group (p < 0.05) at the 10th day after surgery. No marked differences in spike size and peak potential stimulation response time were observed between groups at the 18th day after surgery (p > 0.05).
Discussion
Postoperative cognitive dysfunction is often encountered in clinical anesthesia. Old age and trauma are two important factors; especially the timing of hepatic portal interdiction in elderly patients is different from that in the young. Our research aims to explore how long the blocking time is clinically instructive for patients with super senior age. In this study, we investigated the association among I/R, POCD, and hippocampus in aged rats. Previous studies have proved that POCD is one of the major complications after surgery [16, 17]. In our study, POCD occurred in aged rats with impaired learning and memory abilities according to MWM and passive avoidance tests. More serious cognitive impairments occurred in aged rats with longer ischemic time within 10 days after surgery, indicating that I/R could induce short-term POCD and the severity of POCD was related to ischemic time.
The hippocampal region of the brain plays a crucial role in cognitive functions of learning and memory. PS in the hippocampus is sensitive to ischemia, anoxia, and inflammation of hippocampus. Stimulated Schaffer collateral contributes to the evoked PS generated by a group of synchronous excited CA1 pyramidal cells. Decreased PS peak values indicate the reduced excitatory synaptic interaction between CA1 and CA3 networks. Itoh et al. [18] found that hypercapnia would impair consciousness, leading to a significant reduction of PS amplitude. Similarly, aged rats with impaired cognition after surgery showed the reduced amplitude of PS and prolonged peak potential stimulation response time in our study.
Protein kinase C (PKC) is a phospholipid-dependent enzyme that plays a critical role in activity-dependent neuronal plasticity [19]. PKCγ, as one isoform of PKC, is particularly relevant to learning, memory and is vital to hippocampal function [20–22]. Previous studies showed that PKCγ mutant mice exhibited impairments in long-term potentiation (LTP), in spatial and contextual learning [23, 24]. NF-κB, one of the most important transcription factors, has played a critical role in inflammation and immunity as well as cell proliferation, differentiation, survival and oxidative damage [25, 26]. NF-κB has been proved to be crucial in initiating and regulating the inflammation in nervous system diseases such as Alzheimer’s disease (AD) and Parkinson’s disease [10]. An elevated level of NF-κB was observed in rats with impaired cognition by Zhang et al. [27]. Sakai et al. [28] concluded that NF-κB with increased activity in Kupffer cells promotes inflammatory cytokine expression leading to increased liver inflammation and injury after I/R. In our study, aged rats with a decreased level of PKCγ and elevated level of NF-κB in the I/R group, especially in the I/R (40) group at the 10th day after surgery, are consistent with these studies. It also implied that the changes of PKCγ and NF-κB were closely related to ischemic time in the short-term period. It is well known that vital organ damage can affect the function of remote organ preconditioning. There are many factors influencing the changes of postoperative cognitive function in elderly patients. However, there are no reports on the effects of different degrees of remote organ preconditioning injury on cognition. The goal of this study is to analyze the effects of remote organ preconditioning injury on cognition.
Our study has some limitations. We did not observe the relative pathways among I/R injury, POCD and the hippocampus. More factors should be included in the multi-factorial process. So further investigations are required to support this hypothesis.
In conclusion, I/R injury had a negative impact on the cognitive function of aged rats, led to hippocampus changes with PS abnormity and level changes of NF-κB, PKCγ. However, this cognitive deficit improved over time.