Skin cancers (mostly basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)) are responsible for about 98% of all skin cancers and they are the most common malignancies in the Caucasian population. On the other hand, melanomas account for a small percentage of all skin malignancies, but they are responsible for the majority of deaths due to cutaneous cancers. Although the majority of patients with skin cancer are diagnosed in the early stage of the disease and are cured surgically, still there is a significant number of patients worldwide at stage III–IV disease, for whom other treatment modalities may be necessary. Recent developments and approval of targeted agents and immunotherapy have significantly changed the landscape of the therapy of cutaneous malignancies in the metastatic setting, and they have recently translated into progress in neo-/adjuvant treatment in high-risk locoregional disease [1–5].
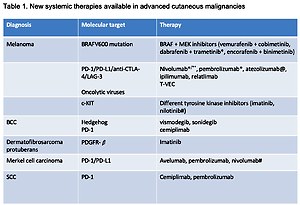
The survival of advanced melanoma and skin carcinoma patients has greatly improved within the last few years. This unprecedented development is mainly related to the introduction of 2 different therapeutic strategies: immunotherapy with use of immune checkpoint inhibitors anti-CTLA4 and/or anti-PD1 in melanoma, SCC, and Merkel cell carcinoma, as well as targeted therapy with kinase inhibitors for BRAF and MEK and hedgehog inhibitors in melanoma and BCC, respectively [1–5]. Table I summarizes all new systemic therapies available for advanced cutaneous malignancies.
Table I
New systemic therapies available in advanced cutaneous malignancies
| Diagnosis | Molecular target | Therapy |
|---|---|---|
| Melanoma | BRAFV600 mutation | BRAF + MEK inhibitors (vemurafenib + cobimetinib, dabrafenib + trametinib*, encorafenib + binimetinib) |
| PD-1/PD-L1/anti-CTLA-4/LAG-3 Oncolytic viruses | Nivolumab*/**, pembrolizumab*, atezolizumab@, ipilimumab, relatlimab T-VEC | |
| c-KIT | Different tyrosine kinase inhibitors (imatinib, nilotinib#) | |
| BCC | Hedgehog PD-1 | Vismodegib, sonidegib Cemiplimab |
| Dermatofibrosarcoma protuberans | PDGFR-β | Imatinib |
| Merkel cell carcinoma | PD-1/PD-L1 | Avelumab, pembrolizumab, nivolumab# |
| SCC | PD-1 | Cemiplimab, pembrolizumab |
The prognosis of patients with melanoma at stage IIB–IV after a complete resection of the lesions is highly heterogenous and related to disease recurrences in 30–70% of cases. Currently, systemic adjuvant treatment with immunotherapy (nivolumab and pembrolizumab) and targeted therapy (dabrafenib and trametinib in BRAF-mutated cases) after surgery in high-risk patients is the standard treatment administered postoperatively with curative intent, leading to decreasing disease relapses at approximately 20% after long-term follow-up [1–5]. New data from 2022 indicate that also the use of adjuvant therapy in earlier stages (IIB-IIC) in high-risk melanomas without nodal metastases statistically significantly improves relapse-free survival (RFS). In the KEYNOTE-716 clinical trial pembrolizumab used postoperatively for up to 1 year in stage IIB or IIC melanoma patients led to a significant reduction in the risk of disease recurrence or death versus placebo, and the RFS rate was improved after 24 months from 72,8% to 81.2% (hazard ratio (HR) = 0.61), but the difference in distant metastasis-free survival (DMFS) was only 6% at this time point [6]. Nevertheless, pembrolizumab has been approved for adjuvant therapy in fully resected stage IIB/IIC melanoma in the European Union, although its use is controversial because the number needed to treat to achieve benefit is 12–13 cases.
A novel strategy in the treatment of locoregional advanced melanoma is the use of systemic immunotherapy preoperatively to further reduce the risk of recurrence and to increase the cure rate. Neoadjuvant immunotherapy is a very attractive strategy because it can be more efficient than adjuvant therapy due to fact that T-cell checkpoint blockade administered preoperatively (when the tumour is not excised) could induce stronger and broader tumour-specific T-cell response in a larger population of lymphocytes. Neoadjuvant immunotherapy is related to a high rate of complete pathological remission. Moreover, patients achieving complete remission after immunotherapy seem to have a durable response as a memory effect of the immune system. The PRADO clinical trial targeted clinical outcomes of 6 weeks of neoadjuvant ipilimumab and nivolumab and the use of pathologic response as a criterion for further treatment personalization, allowing for the omission of therapeutic lymph node dissection (TLND) [7]. Ninety-nine patients with clinical stage IIIB-B and nodal only metastases from cutaneous melanoma were included, and the pathological response rate was 72%, including 61% of cases with major pathological responses. TLND was omitted in 59 of 60 patients with major pathological response, which is related to significantly lower surgical morbidity and improvement of quality of life. The 24-month RFS and DMFS rates were 93% and 98% in patients with MPR and 71% and 76% in patients without pathological responses, respectively. The first randomized clinical trial comparing adjuvant and neoadjuvant strategy in stage III–IV melanoma patients was the SWOG S1801 trial, in which 313 patients were randomized to an adjuvant arm with up to 18 cycles of pembrolizumab or a neo-/adjuvant arm in which 3 cycles of pembrolizumab were given preoperatively. Event-free survival was significantly improved in the neoadjuvant arm compared to the adjuvant arm (HR = 0.58, 0.39 to 0.87; p = 0.004), and the 2-year event-free survival rate was 72% in the neoadjuvant arm versus 49% in the adjuvant arm [8]. The phase III clinical trial NADINA in the stage III melanoma population comparing neoadjuvant and adjuvant therapy is ongoing.
The current results of anti-PD-1 (pembrolizumab or nivolumab) monotherapy indicate a median overall survival (OS) of approximately 2 years, but when the combination of anti-PD-1 and anti-CTLA-4 (nivolumab with ipilimumab) is administered it leads to further improvement of progression-free and OS (recent data indicated for the first time in metastatic melanoma median OS more than 5 years, median OS after 6.5 years was 72.1 months) [9]. Because of sustained activity of these drugs (even after stopping therapy) they are often considered as the gold standard first-line therapy independently of BRAF mutation status. However, the combination of nivolumab and ipilimumab is related to high toxicity with adverse events (mostly immune-related) grade 3/4 at the level of 60%. Thus, new immunotherapy strategies are needed, one of which is the utilization of anti-LAG-3 (lymphocyte-activation gene 3) antibodies because LAG-3 contributes to the mechanism of T-cell exhaustion. A phase 2-3 registration randomized trial compared an anti-LAG-3 drug – relatlimab – combined with nivolumab as a fixed-dose combination to nivolumab alone in patients with previously untreated metastatic or unresectable melanoma [10]. The median PFS in the nivolumab-relatlimab arm was 10.1 months (95% confidence interval (CI): 6.4–15.7) as compared to 4.6 months (95% CI: 3.4–-5.6) with nivolumab monotherapy (HR = 0.75, 95% CI: 0.62–0.92; p = 0.006). The PFS rate at 12 months was 47.7% with relatlimab-nivolumab and 36.0% (95% CI: 30.5–41.6) with nivolumab. Grade 3 or 4 treatment-related adverse events were low and observed in 18.9% of patients in the relatlimab-nivolumab arm, as compared to 9.7% of patients in the nivolumab cohort. The combination of nivolumab and relatlimab has been approved in a fixed-dose for the first-line treatment of advanced (unresectable or metastatic) melanoma in adults and adolescents 12 years of age and older with tumour cell PD-L1 expression < 1%.
The second recently approved drug in metastatic melanoma with a unique mechanism of action is tebentafusp – a new bispecific molecule targeting T cells in the presence of HLA-002. The indication concerns uveal melanoma, and it is designated as an orphan drug. A clinical trial has proven its benefit in terms of OS both compared to historical data (phase II study – median OS 16.8 months) and an active comparator (phase III study – 1-year OS rate 73% vs. 58%, HR = 0.51) [11]. The drug was registered in the European Union in March 2022.
Non-melanoma skin cancers belong to the group of tumours with the highest mutational tumour burden and strong rationale for immunotherapy. There are 2 major achievements in this field in recent months. First, cemiplimab – an anti-PD-1 drug – became the new standard treatment for locally advanced/metastatic basal cell carcinoma after first-line hedgehog inhibitor therapy. A phase II clinical trial demonstrated meaningful activity of cemiplimab and acceptable safety profile in the population of BCC – objective responses were observed in 31% of patients [12]. Even more striking results were achieved in a phase II clinical nonrandomized trial evaluating cemiplimab in neoadjuvant therapy in patients with resectable stage II, III, or IV (M0) cutaneous SCC [13]. In a group of 79 patients receiving cemiplimab preoperatively every 3 weeks with up to 4 doses, pathological complete responses were observed in 40 (51%) patients and MPR in 10 (13%) patients. These results were consistent with imaging responses observed in 54 (68%) patients.