Introduction
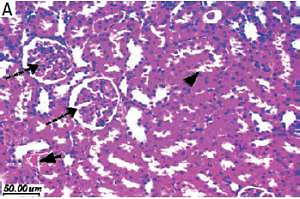
Renal ischemia-reperfusion (I/R) injury is a substantial clinical problem in renal transplantation, which may cause renal dysfunction [1]. The patho-physiological mechanisms of I/R injury are associated with increased expression of intercellular adhesion molecules [2, 3] and pro-inflammatory cytokine [4], leading to acute kidney injury (AKI) [2, 3]. The disturbed intracellular adhesion molecule-1 (ICAM-1) with vascular adhesion molecule leads to activation and trapping of leucocytes. Local cytokines activate leucocytes, which initiate reactive oxygen species (ROS) production, which is a feature of kidney injury in I/R and other chronic kidney diseases [2, 5]. Subsequently, the whole processes end up with tissue swelling and vascular occlusion [2, 6] and enhanced apoptosis and cellular death [1]. Renal I/R injury subsequently leads to pulmonary failure [2, 7].
In contrast, ischemic preconditioning (Ipre) has renoprotective effects by suppressing apoptosis [8] and modulating the cellular cytokine release [9]. Induction of Ipre confers an intrinsic adaptive mechanism and protects against I/R-induced injury [10–12].
Nuclear factor-erythroid-2-related factor 2 (Nrf2), a transcription factor, and its target gene NAD(P)H:quinone oxidoreductase-1 (NQO-1) regulate antioxidant genes, affecting the expression of inducible heme oxygenase-1 (HO-1) [13, 14]. Controlling the inflammatory condition and oxidative stress was shown to have a protective effect through upregulating Nrf2, NQO-1 and HO-I genes in ischemic tissues which reduce apoptosis [15, 16]. The genes also showed a protective effect against chronic kidney diseases [17, 18].
Cilostazol is a calcium sensitizer known by its ROS scavenging effects [19, 20] and suppressing effect on the inflammatory cytokines and apoptotic protein expressions [21].
Levosimendan is another calcium sensitizer, which exhibits anti-aggregatory properties [22] and was shown to reduce AKI [23] through its anti-inflammatory and ROS profiles [24].
As cilostazol and levosimendan were effective in ameliorating renal I/R [25–27], in the present study, we evaluated the protective effect of cilostazol or levosimendan, with and without Ipre, on I/R-induced renal injury and injury of other organs such as the lung. In renal I/R and Ipre, we explored the master regulator of antioxidant gene (Nrf2) expression, and the expression of the antioxidant gene NQO-1, which play a role in both HO-1 expression and ROS production.
Thus, this study aims to demonstrate renal I/R pathophysiology, and the possible reno-protection therapeutic targets of cilostazol and levosimendan.
Material and methods
Seventy adult male albino rats weighing 240–260 g were used. A period of acclimatization was implemented as stated before [28]. The experiments were conducted according to the legal requirements in Poland as well as the National Institute of Health Guide (National Institute of Health Publications No. 80–23, Revised 1978) for the care and use of laboratory animals for experimental procedure, and the institute ethical standards (ethics committee, No. 3324).
Surgery and experimental protocol
Rats were injected with pentobarbital (50 mg/kg/ i.p.), then Ipre and I/R protocols were applied according to Joo et al. [10]. Right nephrectomy was done first, followed by Ipre then I/R inductions. Renal Ipre was induced by placing a microvascular clamp on the left renal pedicle for 5 min, interrupted by 5 min reperfusion for 4 cycles, followed immediately by an I/R maneuver by subjecting the left renal pedicle to 30 min of ischemia and reperfusion for 48 h.
Cilostazol and levosimendan (Sigma-Aldrich Chemical Co., Egypt) were supplied as white and crystalline powders, respectively, and dissolved in normal saline.
To test the effect of cilostazol as an ROS scavenger and levosimendan as anti-aggregatory or its combination in tissue protection, cilostazol (10 mg/kg, twice/day, oral gavage) was administered 2 h [19, 29], and levosimendan (24 μg/kg/day, intraperitoneal) 30 min before the procedures [30, 31].
The animals were divided into seven groups of 10 animals each: (1) the sham group (baseline group): rats were subjected to left renal pedicle exposure with no ischemia, (2) I/R (ischemic reperfusion), (3) Ipre (Ipre + I/R), (4) I/R + cilostazol, (5) I/R + levosimendan, (6) Ipre + cilostazol, and (7) Ipre + levosimendan.
After 48 h of reperfusion, animals were euthanized under anesthesia by exsanguination. The blood samples and a portion of the left kidney and lung tissues were collected and kept at –80°C. The other part was embedded in 10% neutral buffered formalin for histopathological and immunohistochemical assays.
Renal functions
Renal dysfunction was evaluated to determine the effect of I/R, Ipre and the drugs by measuring serum levels of blood urea and creatinine using a Bioclin kit (Santa Coloma, Spain). Urine during 24 h was collected using metabolic cages, then creatinine clearance (CrCl) was calculated as: CrCl = (urine creatinine; mg = dl × urine volume; ml/day)/(serum creatinine; mg = dl × 1440 min) [11].
Arterial blood gas analysis
To study the effect on lung functions, arterial blood samples were obtained from abdominal aorta for pH, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2) and HCO3–measurements [32] with a blood sample analyzer (Roche Diagnostics OMNI C, IN, USA).
Renal and lung oxidative stress levels
Tissue lipid peroxides (LPs) of malondialdehyde (MDA) [6] and glutathione (GSH) levels were measured [28]. The activity of both superoxide dismutase (SOD) [6] and catalase (CATA) [19, 28] was also assessed to evaluate the impact of I/R and the benefits of the Ipre and drugs.
Renal and lung inflammatory assessment
TNF-α, IL-6 and ICAM-1 levels known to be increased with I/R were measured in both renal and lung homogenates using ELISA (BioSource Europe S.A., Brussels, Belgium).
Histopathological evaluation
Both renal and lung damage were evaluated and scored according to Azarkish et al. [7]. The kidney damage was assessed according to the presence of ischemic necrosis, tubular atrophy, hyaline casts and vacuolization. The lung tissue damage was assessed for the presence of congestion, inflammation, and fibrosis. The damage was scored from 1 to 4, where 0 was assigned as normal tissue.
Immunohistochemistry analysis
Rat monoclonal antibodies against apoptotic markers (caspase-3-Bcl-2; Abcam, Cambridge, UK) in renal tissues were used to assess the deleterious effects associated with I/R. The percentage of immunopositive areas was determined using Image J 1.45 F.
Renal antioxidant genes expression
Antioxidant Nrf2, NQO-1 andHO-1 gene expression is sensitive to I/R. Therefore, their levels were assessed from renal tissue, using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) [33]. The gene-specific primer pairs were as follows: for Nrf2, forward primer 5′-ATCCAGACAGACACCAGTGGATC-3′, reverse primer 5′-GGCAGTGAAGACTGAACTTTCA-3′. The (NQO-1) forward primer 5′-AGGCTGGTTTGAGCGAGT-3′, reverse primer 5′-ATTGAATTCGGGCGTCTGCTG-3′; for β-actin, forward primer 5′-TGTTTGAGACCTTCAACACC-3′, the reverse primer 5′-CGCTCATTGCCGATAGTGAT-3′. The (HO-1) forward primer 5′-TGCTCAACATCCAGCTCTTTGA-3′, reverse primer 5′-GCAGAATCTTGCACTTTGTTGCT-3′.
Statistical analysis
Data were analyzed using SPSS, and expressed as mean ± SD. The difference between variables was analyzed using one-way analysis of variance (ANOVA) for quantitative variables, and Kruskal-Wallis test for parameters with non-Gaussian distribution, followed by Tukey’s post-hoc test for multiple comparisons. A difference between groups was considered significant when p-value < 0.05.
Results
Ischemic preconditioning and cilostazol or levosimendan enhanced renal and lung physiological parameters in ischemia-reperfusion
Renal I/R induced an elevation in the serum levels of urea and creatinine associated with a reduction in creatinine clearance (CrCl) compared with the sham-operated group, suggesting I/R-induced renal dysfunction (p < 0.05, Table I). Despite the functional (p < 0.05) recovery associated with the Ipre group in comparison with the I/R group, adding cilostazol or levosimendan to the Ipre group demonstrated more functional recovery (p < 0.05) when compared to the Ipre group. Ipre + levosimendan induced improvement compared to the Ipre + cilostazol treated group (p < 0.05, Table I).
Table I
Effects of ischemic preconditioning, cilostazol or levosimendan and combination of them on renal function in renal ischemia/reperfusion injury
| Groups | Serum creatinine [mg/dl] | Serum urea [mg/dl] | Creatinine clearance [ml/min] |
|---|---|---|---|
| Sham | 0.61 ±0.1 | 26.1 ±2.6 | 1.31 ±0.23 |
| I/R | 2.5 ±0.15¶ | 73.8 ±7.8¶ | 0.13 ±0.03¶ |
| Ipre | 2.1 ±0.17* | 62.0 ±7.4*$ | 0.46 ±0.09¶*$ |
| I/R + cilostazol | 1.8 ±0.13*$ | 56.8 ±3.9*$ | 0.63 ±0.09¶*$ |
| I/R + levosimendan | 1.65 ±0.25*# | 49.1 ±8.1*# | 0.68 ±0.11¶* |
| Ipre + cilostazol | 1.33 ±0.11*# | 45.2 ±4.5*# | 0.82 ±0.18¶*# |
| Ipre + levosimendan | 0.88 ±0.09*#$ | 35.1 ±3.9*#$ | 1.08 ±0.04*#$ |
Rat arterial blood gas analysis was performed to further assess the consequences of renal injury and the protective effect of Ipre and the implemented drugs on lung injury (Table II). The arterial blood pH, PaO2 and PaCO2 values in the Ipre + levosimendan group were improved (p < 0.05) compared to the Ipre and I/R groups.
Table II
Effects of ischemic preconditioning, cilostazol or levosimendan and combination of them on arterial blood gas analysis in renal ischemia/reperfusion injury
| Groups | pH | PaO2 [mm Hg] | PaCO2 [mm Hg] | HCO3- [mEq/l] |
|---|---|---|---|---|
| Sham | 7.35 ±0.02 | 121.4 ±2.75 | 38.20 ±1.27 | 31.25 ±2.97 |
| I/R | 7.17 ±0.03¶ | 92.55 ±2.71¶ | 29.20 ±1.48¶ | 16.88 ±3.03¶ |
| Ipre | 7.19 ±0.03¶ | 93.40 ±1.43¶ | 29.47 ±1.39¶ | 18.18 ±2.54¶ |
| I/R + cilostazol | 7.21 ±0.02¶ | 96.05 ±1.24¶ | 29.07 ±1.69¶ | 17.00 ±2.56¶ |
| I/R + levosimendan | 7.24 ±0.03¶* | 98.23 ±2.24¶* | 31.02 ±1.79¶ | 19.63 ±3.46¶ |
| Ipre + cilostazol | 7.23 ±0.04¶* | 106.8 ±2.89¶*# | 30.43 ±2.15¶ | 20.87 ±3.38¶ |
| Ipre + levosimendan | 7.35 ±0.02¶*#$ | 111.9 ±4.71¶*#$ | 34.62 ±0.66¶*#$ | 26.50 ±3.38*#$ |
Ischemic preconditioning and cilostazol or levosimendan improved renal and lung oxidative stress and inflammatory cytokines
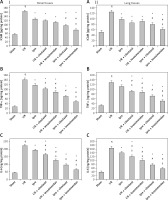
I/R induced an oxidative stress burden associated with an increment of the inflammatory cytokines (p < 0.05) in both renal and lung tissue homogenates in comparison with the sham-operated group (Figures 1 and 2). Ischemic preconditioning either alone or in combination with the implemented pharmacological agents revealed effectiveness with varying degrees in ameliorating these deleterious effects associated with renal I/R. The Ipre + levosimendan treated group exhibited more improvement in these markers compared to the Ipre or Ipre + cilostazol group (p < 0.05) (Figures 1 and 2).
Figure 1
Renal and lung oxidative stress level in the study groups. The effect of ischemic preconditioning (Ipre), ischemia/reperfusion (I/R), cilostazol or levosimendan and combination of them on renal (I) and lung (II) oxidative stress markers, A – MDA, B – GSH, C – SOD, D – CATA. Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to I/R group. #Compared to Ipre group. $Compared to Ipre + cilostazol

Figure 2
Renal and lung inflammatory markers. The effect of ischemic preconditioning (Ipre), ischemia/reperfusion (I/R), cilostazol or levosimendan on renal (I) and lung (II) tissues, A – ICAM, B – TNF-α, C – IL-6. Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to I/R group. #Compared to Ipre group. $Compared to Ipre + cilostazol
Ischemic preconditioning and cilostazol or levosimendan improved renal and lung histopathological changes
The kidney histopathological examination in the I/R group exhibited marked tubular degeneration in comparison with the normal sham group (Figure 3-I A, B). There is focal ischemic tubular necrosis with shrunken glomeruli indicating ischemic necrosis (Figure 3-I B) with an increase (p < 0.05) in the mean histopathological score (Figure 3-II) compared to the sham group. Ischemic preconditioning + the implemented pharmacological agents ameliorated I/R effects (Figure 3-I) compared to the Ipre or I/R group (Figure 3-II; p < 0.05). Parallel damaging changes in the lung tissue was observed (Figure 4) in rats with I/R. Notably, Ipre slightly protected against disrupted alveolar structure, pulmonary interstitial edema and inflammatory cells in the alveolar cavity. However, Ipre + levosimendan had a greater protective effect in lung tissue (Figure 4-II).
Figure 3
Microphotographs of renal tissues of sham, ischemia/reperfusion (I/R), ischemic preconditioning (Ipre), cilostazol or levosimendan groups (H&E 400×). I: Sham (A) showed normal glomeruli and tubular cells (dashed arrows). I/R group (B) showed marked interstitial hemorrhage (arrow), tubular epithelial degeneration (arrowhead) and shrunken glomeruli (dashed arrow) with moderate inflammatory infiltrate. Ipre group (C), I/R + cilostazol (D) and I/R + levosimendan (E) groups showed mild improvement. Ipre + cilostazol treated group (F) showed moderate improvement. Ipre + levosimendan treated group (G) showed preserved renal architecture. II. Histopathological score. Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to I/R group. #Compared to Ipre group. $Compared to Ipre + cilostazol
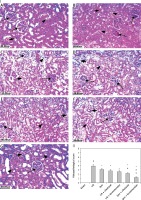
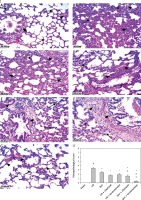
Figure 4
Microphotographs of lung tissues of renal sham, ischemia/reperfusion (I/R), ischemic preconditioning (Ipre), cilostazol or levosimendan groups (H&E 400×). I: Sham (A) showed preserved lung alveoli (dashed arrow), thin walled vessels (arrowhead) and thin bronchioles (arrow) wall. Renal I/R group (B) showed disturbed lung architecture with marked inflammatory infiltrate and congestion (arrowhead), bronchiolar wall thickening. Rats’ lungs of Ipre group (C), I/R + cilostazol (D) and I/R + levosimendan (E) groups showed mild improvement. Ipre + cilostazol treated group (F) showed moderate improvement. Levosimendan with Ipre induced marked improvement. II: Lung histopathological score in renal I/R injury. Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to I/R group. #Compared to Ipre group. $Compared to Ipre + cilostazol
Ischemic preconditioning and cilostazol or levosimendan improved renal apoptotic changes
Figures 5 and 6 show the enhanced renal apoptosis (p < 0.05) in I/R renal tissues as revealed by the elevated pro-apoptotic activator “caspase-3” percentage (Figure 5) and the declined anti-apoptotic protein “Bcl-2” (Figure 6) compared to the sham group. Ipre, cilostazol or levosimendan and the combination of them inhibited apoptosis when compared to the I/R control group (Figures 5 and 6). The Ipre + levosimendan group displayed a reduction in caspase-3 and an elevation in Bcl-2 as compared to the Ipre or Ipre + cilostazol group (p < 0.05) (Figures 5-II, 6-II).
Figure 5
Renal caspase-3 stain microphotographs in different groups (400×). I, A – Sham, B – ischemia/reperfusion (I/R), C – ischemic preconditioning (Ipre), D – I/R + cilostazol, E – I/R + levosimendan, F – Ipre + cilostazol, G – Ipre + levosimendan. II: Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to I/R group. #Compared to Ipre group. $Compared to Ipre + cilostazol
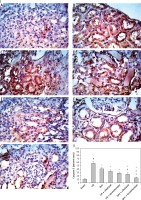
Figure 6
Renal Bcl-2 stain microphotographs in different groups (400×). I, A – Sham, B – ischemia/reperfusion (I/R), C – ischemic preconditioning (Ipre), D – I/R + cilostazol, E – I/R + levosimendan, F – Ipre + cilostazol, G – Ipre + levosimendan. II: Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to I/R group. #Compared to Ipre group. $Compared to Ipre + cilostazol
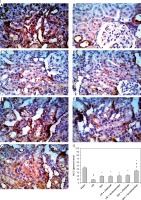
Ischemic preconditioning and cilostazol or levosimendan enhanced antioxidant genes
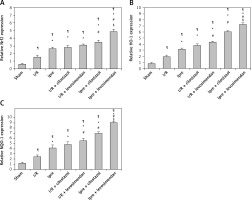
Renal expression of Nrf2, NQO-1 and HO-1 genes was strengthened by Ipre in comparison with either the sham-operated or I/R control group (p < 0.05, Figure 7). Adding the implemented pharmacological agents to Ipre revealed effectiveness in enhancing expression of these genes compared to the Ipre group. It was evident that Ipre + levosimendan strengthened the expression of these genes compared to the Ipre + cilostazol treated group (p < 0.05, Figure 7).
Figure 7
Renal expression of Nrf2/HO-1/NQO-1 assessed by PCR in different groups. A – Nrf2, B – HO-1, C – NQO-1. Values are mean ± SD (n = 8–10), analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ¶,*, #, $P < 0.05. ¶Compared to sham group. *Compared to ischemia/reperfusion (I/R) group. #Compared to ischemic preconditioning (Ipre) group. $Compared to Ipre + cilostazol
Discussion
Renal I/R injury is the consequence of many functional and dynamic processes, in which the underlying pathophysiological mechanisms revolve around oxidative stress that starts in the kidney and ends in lung inflammation [34, 35]. In contrast, Ipre has a renal protective effect [8], which protects the kidney by activating the antioxidant Nrf2 defense gene expression [33]. Augmentation of Ipre by levosimendan and cilostazol provided a further step in renal and lung protection against I/R.
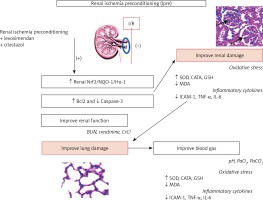
Figure 8 summarizes the findings as follows: Ipre combination with cilostazol or levosimendan was associated with upregulated expression of renal antioxidant genes (Nrf2/HO-1/NQO-1). This is associated with a decrease in renal apoptosis and oxidative stress burden and an improvement in renal and lung histopathological and functional parameters.
Figure 8
Schematic representation of ischemic preconditioning (Ipre) pathophysiology and its augmentation by cilostazol or levosimendan. Summary of the ROS, inflammatory and gene expression changes are shown, with the protective effect of Ipre combined with cilostazol or levosimendan on renal ischemia/reperfusion (I/R) injury
Ischemia/reperfusion injury associated with the restoration of blood flow and re-oxygenation [10] initiates ROS and pro-inflammatory cytokine production [2, 6, 9, 34]. The phenomenon known as Ipre was shown to enhance renal cellular resistance to ischemia after hypoxic preconditioning [36]. Different Ipre models are proved to partially protect the kidney against I/R injury [8, 10, 33]. However, both cilostazol and levosimendan, by their antiplatelet and anti-inflammatory properties [22, 26, 37–39], enhanced Ipre- induced renal and lung protection, revealed by the improvement of the renal and lung functional and structural parameters.
Local renal I/R injury proved to have deleterious effects on the lung [40], as the current study confirmed. Therefore, in the I/R group, acidosis and impaired oxygenation were detected [32]. This deleterious effect was improved with Ipre and augmented by cilostazol or levosimendan via their ameliorating effect on the lung inflammation and oxidative stress as was evident in the current study and previous studies [34, 35]. The released renal inflammatory cytokines affecting the lung [1, 4, 41] were ameliorated by Ipre with cilostazol or levosimendan through their systemic anti-inflammatory and antioxidant effects [17, 19, 24, 38, 42]. Renal ischemia induces endothelial damage with endothelial-leukocyte cell adhesion, causing blockage in microvascular blood flow [2] and an increase in the ICAM protein expression, as evident in the present results, which was reduced with Ipre, cilostazol or levosimendan treatment. ICAM upregulation is a key marker for the endothelial-leucocyte adhesion processes [24], releasing the pro-inflammatory cytokines TNFα and IL-6, which supports the present observations [2]. The released cytokines enhance leukocyte sequestration [43], which yield more renal injury through ROS production [44]. The present study also revealed similar changes in the pulmonary ICAM expression. Released renal inflammatory mediators could affect pulmonary microvascular endothelial cells, which are a potential link between the kidney and lung injury [32, 34]. Cilostazol and levosimendan have been shown to improve the inflammatory and oxidative stress burden in heart and lung tissues [24, 45–47]. Therefore, AKI ramification on kidney and lung can be attenuated by modulation of the I/R-induced inflammatory response.
Ipre augmented by cilostazol or levosimendan decreased renal apoptosis in response to I/R injury [24, 39], thus favoring cell survival. Moreover, Ipre is associated with stimulation of the key regulator of the Nrf2 antioxidant defense gene and its two related antioxidant genes, HO-1 and NQO-1 [12, 17, 48]. Ipre augmented-Nrf2/HO-1/NQO-1 expression, which was enhanced by Ipre + levosimendan or cilostazol, playing a major role in the detoxification of ROS [48]. The upregulation of the antioxidant gene battery has therapeutic and clinical applications [49], and enhances understanding the pathophysiology of renal I/R [50].
In conclusion, cilostazol and levosimendan enhanced the renoprotective effects of Ipre against acute renal I/R injury. The fundamental mechanisms underlying the positive influence of Ipre and drug treatment might be due to activation of the endogenous adaptive renoprotective gene Nrf2, and its dependent antioxidant genes HO-1 and NOQ1, with suppression of oxidative stress, inflammatory response, and apoptotic cell death. The summation of these processes has local protective effects on the kidney and remote effects on the lung. This helps in understanding the pathophysiological mechanisms associated with I/R and the key role of cilostazol and levosimendan in treatment of acute and chronic renal diseases.