The epidemic of lipid disorders in Poland and its consequences
Hypercholesterolemia is the most common cardiovascular (CV) risk factor in Poland, affecting as many as 21 million people [1, 2]. Unfortunately, most of those affected are not aware of this, and many still ignore the risk associated with elevated cholesterol. Of the remaining patients, many are not treated optimally, and only about a quarter reach low-density lipoprotein cholesterol (LDL-C) targets, including only 17% of patients at very high CV risk [3, 4].
An equally important challenge, and an even larger unmet need, is associated with dyslipidemia related rare diseases, including familial hypercholesterolemia (FH). The prevalence of heterozygous FH (HeFH) in the Polish population is estimated to be 1 : 250, which corresponds to 150 thousand HeFH patients, of which only a small proportion have been diagnosed (~5%) and even fewer receive appropriate treatment [5, 6]. Early diagnosis and optimal therapy are critically important for these patients, as they might be at even 100-fold higher risk of occurrence of atherosclerotic cardiovascular disease (ASCVD) and have a 3–5 higher risk of death [7].
Cardiovascular disease is still the leading cause of mortality worldwide, and as many as 75% of all deaths may be attributable to ASCVD [1]. According to the 2020 Health Needs Maps (data for 2019) in Poland, ischemic heart disease (IHD) is diagnosed each year in 85,753 people (223.1 per 100,000 people), and the morbidity is 1,491,616 (3,880.9 per 100,000 people) [8]. The number of IHD deaths in 2019 was 97,188 (252.9 per 100 thousand people). Ischemic strokes were diagnosed in 74,455 people (193.7 per 100,000 people), with the prevalence 623,986 (117.4 per 100,000 people), and the number of deaths in 2019 was 45,104 (117.4 per 100 thousand people). Finally, the incidence of peripheral arterial disease (PAD) in the same investigated period was 85,157 people (221.6 per 100,000 people), the incidence was 938,059 (2,440.7 per 100,000 people), and the number of PAD-related deaths was 1,141 (3.0 per 100 thousand people) [8]. It needs to be emphasized that during the coronavirus pandemic, the above numbers have essentially worsened, creating a large health debt, what was associated with lack of (or late) diagnosis, insufficient disease monitoring, and lack of suitable pharmacotherapy and delayed cardiovascular interventions (including angioplasty) [9]. Therefore, an urgent call for action is now necessary, including ensuring the widespread availability of innovative therapies to mitigate this health debt and prevent further needless deaths.
Inclisiran – a new effective therapeutic option in the treatment of hyperlipidemia
Inclisiran is a first-in-class cholesterol-lowering double-stranded small interfering ribonucleic acid (siRNA) conjugated on the coding strand to N-acetylgalactosamine (GalNAc) to facilitate its uptake by hepatocytes [10]. It binds to the asialoglycoprotein receptors (ASGPRs) there, which are highly expressed only in hepatocytes, a fact which explains the very good safety profile of this drug. Inclisiran uses the mechanism of RNA interference and directs the catalytic breakdown of PCSK9 mRNA [10, 11]. This increases the recycling and expression of LDL-C receptors on the surface of hepatocytes, which enhances LDL-C uptake and effectively reduces circulating LDL-C levels [10, 11].
Several phase I–III studies with inclisiran have already been completed, while other phase III studies are still ongoing under the ORION and VICTORION clinical development programs (the details are available elsewhere [12]). The available results (mainly based on the pooled analysis of ORION 9–11 studies) clearly show that inclisiran very effectively reduces LDL-C by about 52–55% (and about 44-48% in patients with FH), enabling LDL-C targets to be achieved in 76% and 58% of investigated patients in high and very high-risk patients, respectively [12–14]. The analyses also showed that inclisiran effectively reduced non-HDL-C, apolipoprotein B, and lipoprotein(a) (Lp(a)) by 42.8, 40.2, and 20%, respectively, thereby demonstrating good efficacy in the management of residual risk [12–14]. This is especially important now, as in the recent Polish guidelines, non-HDL is considered as an equal important lipid parameter to LDL-C, and strong recommendations were also suggested for Lp(a) measurement (for all ASCVD and FH patients with IIbC level of recommendations), which is still assessed very rarely in Poland (mostly in patients at borderline CVD risk and in those with premature myocardial infarction) [15].
There are still no data on the effect of inclisiran and cardiovascular outcomes, and the ORION-4 cardiovascular outcomes trial (CVOT) is estimated to be completed in 2026 (based on the information from ClinicalTrial.gov) [16]. However, in our opinion these results are not necessary to start reimbursing inclisiran within the drug program, in light of an overwhelming body of data indicating a strong link between LDL-C reduction and effective prevention of CVD events and death [17]. Moreover, the first meta-analyses based on the available data (ORION 9–11 studies) showed that we might expect a reduction of major adverse cardiac events (MACE) by as much as 24–30% [18, 19]. If we compare this with the 15% reduction of the primary endpoint observed in the FOURIER trial with evolocumab and ODYSSEY OUTCOMES with alirocumab, with the predictions that had been at the same level as currently for inclisiran (also bearing in mind that the population in the PCSK9 inhibitor CVOT were at higher CVD risk – acute coronary syndrome (ACS) patients), the 15–20% reduction of the main endpoint with inclisiran (MACE defined as time to first occurrence of coronary heart disease (CHD) death or myocardial infarction or fatal or non-fatal ischemic stroke or urgent coronary revascularization procedure) would represent a very important clinical success [16, 20].
It is also worth emphasizing that, considering the design of the ORION program, inclisiran has also demonstrated effectiveness and safety in primary prevention (HeFH patients were included in ORION-9, and ASCVD risk equivalent ones with HeFH, diabetes, those at high CVD risk (10-year risk of a CV event of ≥ 20% as assessed by the Framingham Risk Score) in the ORION-11 trial) [13, 14]. Also, patients with chronic kidney disease (CKD) stage 4 (with eGFR within 15–29 ml/min/1.73 m2), based on the data from the ORION-7 study, might benefit from inclisiran [21]. This is a group which is one of the greatest challenges for cardiologists and lipidologists in the effective management of lipid disorders, as there is currently no indicated lipid-lowering therapy [15, 22].
We have already emphasized the unique safety profile of inclisiran [11–14]. The only adverse reactions related to inclisiran were injection site adverse reactions (8.2%); however, the vast majority of these symptoms were mild, transient and did not require discontinuation of treatment [11–14].
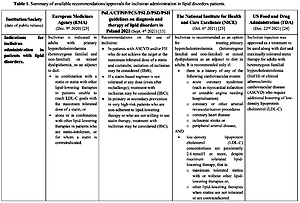
Inclisiran was approved by the European Medicines Agency (EMA) on 9 December 2020 [23] and a year later (22 December 2021) by the US Food and Drug Administration (FDA) [24, 25]. The indications for inclisiran administration based on different agencies and recommendations are presented in Table I.
Table I
Summary of available recommendations/approvals for inclisiran administration in lipid disorder patients
| Institution/Society (date of public release) | European Medicines Agency (EMA) (Dec. 9th 2020) [23] | PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021 (Sept. 4th 2021) [15] | The National Institute for Health and Care Excellence (NICE) (Oct. 6th 2021) [25] | US Food and Drug Administration (FDA) (Dec. 22nd 2021) [24] |
|---|---|---|---|---|
| Indications for inclisiran administration in patients with lipid disorders | Inclisiran is indicated in adults with primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia, as an adjunct to diet:
| Recommendations on the use of inclisiran:
| Inclisiran is recommended as an option for treating primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia as an adjunct to diet in adults. It is recommended only if:AND | Inclisiran injection was approved as a treatment to be used along with diet and maximally tolerated statin therapy for adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of low-density lipoprotein cholesterol (LDL-C) |
Key points for reimbursement of inclisiran in Poland
Based on all of the data above, we strongly believe that inclisiran should be immediately available for very high-risk patients in Poland, as only 1/6 of these patients (17%) currently reach their LDL-C target [3]. We think that the available data from the ORION program on the high efficacy and unique safety (despite the indirect data on cardiovascular outcomes) are enough to add inclisiran to the existing drug program B-101 for PCSK9 inhibitors. There are several reasons to justify such an approach:
Despite the fact that the drugs act through different mechanisms to inhibit the PCSK9 protein, the target is the same, and in the scientific literature both classes are referred to as ‘PCSK9 targeted approaches’ [26];
Despite some differences in the PROFICIO, ODYSSEY and (VICT)ORION programs, for the most part, we observe similarities, especially in the group of patients being investigated (FH, ASCVD, special populations) [11, 15, 20];
The effect on the lipid profile (not only on LDL-C but also on non-HDL, apoB, and Lp(a)) also seems to be similar with a slightly (a few %), clinically irrelevant, greater efficacy of PCSK9 inhibitors in the reduction of LDL-C, but with better safety of inclisiran (also in respect of the response to therapy, which is 100% for this drug) [11–15, 20];
Having three drugs in the drug program would also mean greater availability of innovative, very effective therapy for the patients with the largest unmet needs in Poland – those with HeFH and after ACS.
In our opinion, such an approach is not only effective (especially if the National Health Fund finally announces competition procedures for new centers – mainly interventional cardiology ones – which include patients with early ACS), but also the fastest approach (the alternative is a new drug program, for which the approval process would be much longer and probably less effective). Taking this into account, we would also like to apply for some necessary amendments to the existing drug program for PCSK9 inhibitors (B-101), to ease the process of patients’ qualification and increase their number in both arms (as of March 2022 in both arms there are only about 350 patients included, mostly in the FH arm). Therefore, we would like to recommend the following amendments to the existing drug program, considering its possible extension for inclisiran:
– There is a great need to have a clear definition of statin intolerance based on existing recommendations, including the most recent guidelines of six Polish scientific societies [15] and recent recommendations of the International Lipid Expert Panel (ILEP) [27]. We, therefore, recommend using the practical definition of statin intolerance as ‘documented intolerance to at least two statins, including the second statin with the lowest dose recorded, for a period of at least 3 months’;
– In the current version of the drug program the early (epidemiologically) acute coronary syndrome was defined based on the data from the ODYSSEY OUTCOMES trial (within 12 months) [20], but there are also strong data available on both the very high risk of recurrent CVD events and the effectiveness of PCSK9 targeted therapy for early ACS defined as within 24 months (in the ORION program no such time limits are applied) [20, 28, 29]. This may be of particular importance in Poland, as many patients after ACS are automatically included in the comprehensive coordinated care after myocardial infarction (KOS-Zawał) for only 12 months after an event [30]. So, the possibility of also including these patients at very high and extremely high risk for over 12 months after an event would be expected to be of benefit for them;
– Finally, with respect to the existing program, we would like to recommend supplementing the ‘Criteria for exclusion in the program’ with the possibility of inclusion of patients with chronic kidney disease and eGFR 15–29 ml/min/1.73 m2, who might be treated with inclisiran [21]. This might be indeed a great benefit for patients with kidney impairment, which is common in ASCVD patients [15] (the updated version of the B-101 program with the above changes is presented in Table II).
Future perspectives
We would like to emphasize that the above suggestions are only a kind of compromise between the existing data, needs, and healthcare system and payer possibilities, in order to enable inclisiran to be reimbursed as soon as possible for patients at a very high CVD risk. We are aware, however, that this is merely the first step towards establishing the final population of patients that should be treated with these innovative PCSK9 targeted therapies. We should also do our best to improve the effectiveness of the commonly available and cheap therapies, including high intensity statin therapy, and especially the combination therapy of statins and ezetimibe (including as an upfront therapy), which is severely underused in Poland [3, 7, 15, 31].
Table II
Experts’ suggestions on the extension of the existing therapeutic program B-101 (with inclisiran inclusion): PCSK9 inhibition in patients with lipid disorders (ICD-10 E78.01, I21, I22, I25)
We have, therefore, already completed the first part of the report on the group of patients that might benefit the most (with the detailed calculation of the group sizes and budget impact), based on the available data but also the characteristics of the Polish healthcare system [32]. This might be the next step to be considered in the extension of the group of patients on these innovative therapies. This includes, among others: (1) reduction of the required level of LDL-C from 100 (2.5 mmol/l) to at least 70 mg/dl (1.8 mmol/l) for ACS patients and without the level limit (LDL-C ≥ 55 mg/dl/1.4 mmol/l) for those with two myocardial infarctions (MIs), (2) separation of the group of patients after two MIs and multivessel coronary artery disease (MVCAD) into subjects with two MIs and those with ACS and MVCAD, who are already identified as having extremely high cardiovascular risk (it seems to be a mistake in the drug program from the beginning) [15, 33, 34], (3) inclusion in the drug program of patients with premature myocardial infarction (men ≤ 55 and women ≤ 60 years of age). In our opinion, future changes should also consider patients with a high level of lipoprotein(a) (especially in light of the first Polish epidemiological data on the number of patients with elevated Lp(a) levels), as well as those in primary prevention at high CVD risk (we have such data in the (VICT)ORION program), especially as this is in line with the recommendations for therapy with PCSK9 inhibitors in primary prevention in the European and Polish guidelines [15, 35].
Conflict of interest
Maciej Banach: speakers bureau: Amgen, Herbapol, Kogen, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo-Nordisk, Sanofi-Aventis, Teva, Zentiva; consultant to Amgen, Daichii Sankyo, Esperion, Freia Pharmaceuticals, Novartis, Novo-Nordisk, Polfarmex, Sanofi-Aventis; Grants from Amgen, Mylan/Viatris, Sanofi and Valeant; CMO at Nomi Biotech Corporation Ltd; Jarosław Kaźmierczak: lecture fees and Advisory Boards: Amgen, Novartis, Sanofi; Przemysław Mitkowski: speaking fees: Novartis, Pfizer, Servier, consultant fees: Novartis, Sanofi-Aventis; Marlena Broncel: speaking fees: Amgen, Mylan/Viatris, Novartis, Novo-Nordisk, Polpharma, Sanofi-Aventis, Servier, Teva, Zentiva; Mariusz Gąsior: lecture fees and Advisory Boards: Amgen, Novartis, Sanofi-Aventis. Marek Gierlotka: speakers bureau: Novartis, Bayer, Sanofi, Orion Pharma, Astra Zeneca, Boehringer Ingelheim; Robert Gil: speakers bureau: Novartis; Piotr Jankowski: Honoraria and grants: Boehringer-Ingelheim, Novartis, NovoNordisk, Sanofi, Servier, Zentiva; Maciej Niewada: speaking or consultation fees apart from honorarium for HTA dossiers: Novartis, Novo-Nordisk, Polpharma, Sanofi-Aventis, Servier, Biogen, Amgen, EverPharma, Polfa Tarchomin, Jansen; Adam Witkowski: speaking fees and Advisory Boards: Amgen, Novartis, Sanofi; all other authors have no conflict of interest.