Introduction
Acute lung injury (ALI) is one of the clinically critical and serious diseases. It is often related to microbial infection, injury and other factors and is difficult to treat clinically, and it has a high mortality rate. Sepsis caused by Gram-negative bacterial infection is the most common cause of ALI. The mechanism may be related to lung inflammation exudation and impaired gas exchange function. Various signaling pathways are involved, making the mechanism complex, so the exact mechanism is not fully understood. Lipopolysaccharide (LPS), as a main component of the cell wall of Gram-negative bacteria, is the main pathogen of infectious diseases [1]. It can activate a large number of inflammatory cells, induce acute inflammation and lead to the occurrence of ALI.
The main inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the brain is widely present in mammals and is one of the important neurotransmitters in the lung [2]. Studies have shown that GABA not only is involved in the process of proliferation, regulation, differentiation and maturity of epithelial cells [3, 4], but also may improve mucosal immune function, anti-aging, anti-tumor and other effects under the mediation of the GABA receptor [5, 6]. In recent years, studies have shown that GABA has a certain therapeutic effect on ALI, but its specific mechanism has not been fully elucidated. In this experiment, ALI models were established by LPS. The related mechanism of GABA protecting ALI in mice was explored by detecting the levels of various inflammatory factors in bronchoalveolar lavage fluid (BALF) and lung tissues.
Material and methods
Animals and grouping
128 healthy male 6–8-week-old C57BL/6 strain mice, weighing 18-22 g, were purchased from Beijing Viewsolid Biotechnology Co. Ltd. The experimental mice were raised in cages in a specific pathogen-free (SPF) environment to ensure adequate food and water, and the laboratory temperature was maintained at around 25°C.
Construction of ALI models of the mice
Forty-eight mice were taken and anesthetized by intraperitoneal injection of 1 g/kg 4% chloral hydrate. They were randomly divided into the following 4 groups, with 12 mice in each group: control group (0.2 ml normal saline for intraperitoneal injection), LPS group (LPS 30 mg/kg for intraperitoneal injection), GABA + LPS group (GABA 10 mg/kg was given 1 h before LPS intraperitoneal injection), dexamethasone (Dex) + LPS group (Dex 5 mg/kg was given 1 h before LPS intraperitoneal injection). GABA was purchased from Tianjin Chase Sun Pharmaceutical Co., Ltd.; LPS (E.coli055: B5) and Dex was purchased from Sigma. The mice were anesthetized 6 h after LPS or saline injection. Then they were sacrificed. BALF and lung tissues were collected and various indexes were measured.
Assessment of survival rates of the mice
In order to observe the protective effect of GABA on ALI, the same as above, 80 mice were taken and divided into 4 groups, with 20 mice in each group. The mental state was detected using the tail suspension test to record the quit resistance time. The survival situation of each group of mice was recorded after LPS or normal saline injection. They were observed once every 8 h for a total of 72 h. The survival rate was calculated and the survival curve was drawn.
Total number, differential counting and protein content assaying of BALF inflammatory cells
The mice were fixed, and their skins and muscles in the median necks were cut to expose neck tracheae. Tracheal intubation was performed, and their lung tissues were lavaged 3 times with 0.4 ml of physiological saline, and then BALF was collected at the 3 times of combination. After centrifugation at 3500 rpm for 10 min at 4°C, the precipitate was resuspended in 1 ml of red blood cell lysate to remove red blood cells; then, after the second centrifugation at 3500 rpm for 4 min at 4°C, the precipitate was resuspended in 100 μl of saline for white blood cell count; after Wright-Giemsa staining, differential counting of the cells was performed [7]. The supernatant was taken, and its protein content was measured with the BCA protein concentration assay kit, and the severity of inflammatory exudation in each group of mice was compared.
Levels of inflammatory factors TNF-α, IL-1β, IL-10 and MPO in lung tissues were detected by ELISA
Approximately 100 mg of lung tissues was homogenized in 1 ml of pre-cooled PBS (pH = 7.4), and centrifuged at 12000g, for 10 min at 4°C. The levels of tumor necrosis factor α (TNF-α), interleukin (IL) 1β, IL-10 and myeloperoxidase (MPO) in the lung tissues were measured by reference to the ELISA kit instructions. MPO assay kit was purchased from Hycult Biotech and TNF-α, IL-1β and IL-10 ELISA assay kits were purchased from R&D Systems.
Lung tissue wet/dry weight ratio (W/D)
After the mice were fixed, their chests were opened, and their left lung lobes were washed twice with normal saline. After the blood on the surface was absorbed by filter paper, the wet weight of the lung tissue was weighed; it was baked in a 70°C incubator for 72 h until the dry weight was constant, in order to weigh the dry weight; then the wet/dry weight ratio was calculated. W/D represents water contents of the lung tissues and is used to assess the severity of pulmonary edema.
Pathological observation of the lung tissues
After the mice were sacrificed, the gross specimens of the lungs were dissected and observed. The fresh tissues of the left lower lobes of the mice were taken and fixed in 4% paraformaldehyde for 24 h; they were dehydrated in a gradient by alcohol, dewaxed and embedded in paraffin, in order to make the sections with thickness of 4 μm; the pathological changes of the lung tissues were observed under a light microscope after HE staining. By reference to the method of Mikawa et al. [8], the degree of lung injury was evaluated according to the four categories “lung tissue congestion, edema, interstitial inflammation and inflammatory cell infiltration”; according to the severity of each category, the degree of lung injury was divided into level 0 (normal), level 1 (mild), level 2 (moderate), level 3 (severe) and level 4 (extremely severe), and the total lung injury score was calculated by the scoring and addition for the four categories.
Immunohistochemistry assay
The lung tissue sections were fixed on slides with 4% paraformaldehyde for 15 min, and the slides were immersed and washed in phosphate buffered saline (PBS) 3 times, 3 min each time. Normal goat serum was added dropwise to the slides and blocked at room temperature for 30 min. Then, a sufficient amount of the diluted primary antibody was added dropwise to each slide, placed in a wet chamber, and incubated at 4°C overnight. The next day, the slides of the cells were immersed and washed 3 times with PBST, 3 min each time; the diluted HRP-labeled secondary antibody was added dropwise and incubated at 37°C for 1 h in a wet chamber; the sections were immersed 3 times with phosphate buffered solution (PBST), 3 min each time. Diaminobenzidine (DAB) was added dropwise, and incubated for 30 s in the dark. The slides were sealed with a slide sealing liquid and then images were observed and collected under a light microscope.
Western blot
The protein was extracted using RIPA buffer and centrifuged at 4°C for 25 min. The protein contents were determined by the BCA method. The samples were separated by 12% SDS-PAGE and transferred to PVDF membrane. Rabbit anti-human GABA type A receptor or type B receptor antibody (1 : 200, Abcam, Shanghai, China) was added overnight at 4°C after the membrane was blocked by 4% BSA for 1 h. Then secondary antibody (1 : 1000) was added at room temperature for 1 h. An enhanced chemiluminescence (ECL) kit (Pierce, IL USA) was used for luminescence development [9].
Ethics
All animal experiments and procedures were approved by the Institutional Animal Care and Use Committees of Shandong University, China (SDIU-2016443).
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software. Quantitative data were expressed as mean ± standard deviation (x ± SD), one-way analysis of variance (ANOVA) was used for comparison between groups, and the q-test was used for comparison between groups. The survival rate was calculated by Kaplan-Meier curve, and the survival curves of different groups were compared by the log-rank test. P < 0.05 showed that the difference was statistically significant.
Results
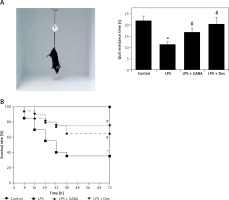
Survival rates of the mice
The mice of the control group were active, breathing evenly and with shiny hair, while the mice of the LPS group were in poor spirits, showing markedly reduced activity, with purulent exudation in the corners of their eyes; some of them had diarrhea and nosebleeds, accelerated breathing, cowering legs, slow response and messy hair. The general mental state of the LPS + GABA group and the LPS + Dex group was significantly better than that of the LPS group. The tail suspension test was used to detect the mental state of mice in each group, and the time of “Quit resistance” was recorded. It was found that the quit resistance time of LPS was shortened, while the quit resistance time was significantly prolonged after GABA or Dex treatment (Figure 1 A). After modeling, the survival time of each mouse was recorded, and the survival analysis curve was drawn. The results are shown in Figure 1 B. Deaths occurred mainly during the 24th h to the 72nd h, and deaths were less after the 72nd h. At the same time, the log-rank method was used to compare the survival rates among different groups. The results showed that there was no mouse death in the control group, and the survival rate of the LPS group was significantly lower than that of the control group (p < 0.05). GABA and Dex could significantly improve the survival rate of ALI mice induced by LPS (p < 0.05).
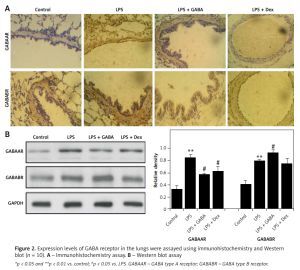
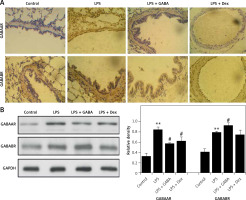
Expression of GABA receptors in the lungs
Next, it can be observed that immunoreactive reactions of GABAA and GABAB receptors are mainly localized in the cytoplasm of airway epithelial cells, showing a brownish-yellow sporadic expression. In the ALI models, the expression of GABAA and GABAB receptors was significantly enhanced. After GABA treatment, the expression of GABAA receptor was significantly decreased, and GABAB was significantly enhanced (Figure 2 A). The results of Western blot showed similar trends, indicating that the inflammatory response can be inhibited effectively by regulating the balance of type A and B receptors (Figure 2 B).
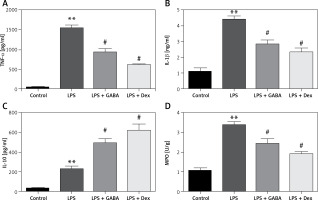
Expression of TNF-α, IL-1β, IL-10 and MPO in lung tissues
The levels of TNF-α and IL-1β in the lung of the LPS group were significantly higher than those in the lung of the control group (p < 0.01); GABA and Dex could significantly down-regulate the levels of TNF-α and IL-1β in lung (p < 0.05). The difference in TNF-α and IL-1β levels between the LPS + Dex group and the LPS + GABA group was not statistically significant (Figures 3 A, B). Moreover, the IL-10 level in the lung of the LPS group was significantly higher than that in the lung of the control group (p < 0.01), which was significantly higher than that in the lung of the LPS group after GABA treatment (p < 0.05, Figure 3 C). The MPO level in the lung tissues of the LPS group was significantly higher than that in the lung tissues of the control group at 6 h after modeling (p < 0.01), which was significantly lower than that in the LPS group after GABA treatment (p < 0.05, Figure 3 D).
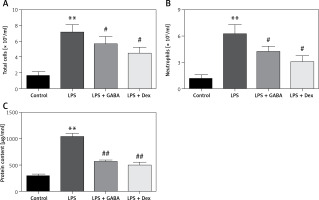
Changes in total number of cells, number of neutrophils and protein content of inflammatory cells in BALF
The total number of cells, the number of neutrophils and the level of protein in BALF of the LPS group were significantly higher than those in the control group (p < 0.01), which were significantly decreased in the LPS + GABA group and the LPS + Dex group (p < 0.05). There was no significant difference between the LPS + GABA group and the LPS + Dex group (Figures 4 A–C).
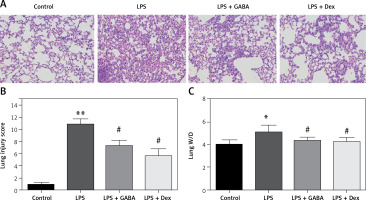
Pathological changes in lung tissues
After HE staining in lung tissues of the mice, there were no inflammatory cells infiltrated in the lung tissues of the control group. However, in the LPS group, there were disordered alveolar structure, alveolar capillary exudation and hemorrhage, massive inflammatory cell infiltrated in the pulmonary interstitium and alveolar spaces, thickened alveolar walls and some collapsed alveoli. Hemorrhagic exudation of pulmonary interstitium and alveoli in the LPS + GABA group and the LPS + Dex group was less than that in the LPS group (Figure 5 A). The scores of pulmonary histopathology (Figure 5 B) showed that the scores of the LPS group were significantly higher than those of the control group (p < 0.01), and the scores of the LPS + GABA group and the LPS + Dex group were significantly lower than those of the LPS group (p < 0.05). The results of lung tissue W/D weight showed that the lung tissue W/D weight ratio in the LPS group was significantly higher than that in the control group (p < 0.05, Figure 5 C) and significantly lower than those in the LPS + GABA group and the LPS + Dex group (p < 0.05). There was no significant difference in lung tissue W/D ratio between the LPS + GABA group and the LPS + Dex group (p > 0.05).
Discussion
Sepsis is a systemic inflammatory response caused by infection that can lead to multiple organ failure. Lung tissue is one of the most important target organs. So far, the pathogenesis of ALI/acute respiratory distress (ARDS) has not been very clear. The inflammatory mechanism mediated by the innate immune response may play a leading role. Neutrophil infiltration is the main marker of inflammation in the lungs during ALI. By releasing cytotoxic substances such as proteases and peroxides and a large number of inflammatory factors, it can damage capillary endothelial cells and alveolar epithelial cells and activate adjacent neutrophils to cause an inflammatory response of cascade amplification [10]. As a commonly used non-specific anti-inflammatory drug, Dex can significantly inhibit local and systemic inflammatory responses in patients with ALI, and improve patients’ clinical symptoms and long-term prognosis, but its side effects are severe. Therefore, it is necessary to develop new and effective compounds that can replace hormone therapy in order to prevent systemic adverse reactions.
In this study, it was found that GABA alleviated the inflammatory response in ALI mice, improved the mental state of the mice, prolonged the survival time of the mice, and reduced the lung tissue damage caused by LPS and the infiltration of neutrophils and other inflammatory cells in their lungs; thereby a positive feedback path for cascade reactions of waterfall-like inflammation was blocked. LPS initiates a downstream signaling cascade reaction through toll-like receptor-4 (TLR-4) on the cell surface and induces gene expression of pro-inflammatory cytokines such as TNF-α and IL-1β. IL-10 is a Th2-cell-derived anti-inflammatory cytokine, which can effectively inhibit and terminate the inflammatory response, inhibit the release of inflammatory cytokines TNF-α, IL-1β, etc., and inhibit the immune inflammatory response. The results of this study showed that GABA significantly down-regulated the expression of TNF-α and IL-1β, up-regulated the expression of IL-10, and then maintained the relative balance between pro-inflammatory factors and anti-inflammatory factors. Thereby it is beneficial to the prognosis and outcome of ALI patients. MPO acts as a hallmark enzyme for neutrophils and its activity reflects the accumulation of neutrophils in lung tissue [11]. The activity of MPO was significantly increased when ALI occurred. Most drugs that control the inflammatory response of ALI can reduce the level of MPO. In this experiment, the application of GABA pre-protection significantly down-regulated the level of MPO in the lungs of the mice, suggesting that GABA attenuated LPS-mediated lung injury by inhibiting polynuclear neutrophil aggregation. Another characteristic of ALI is that a large amount of fluid is exuded into the alveolar space, which makes the alveolar-capillary barrier function impaired and results in alveolar edema and reduction of gas exchange efficiency. In order to determine the severity of pulmonary edema and lung permeability in mice, the lung tissue W/D ratio was measured in this experiment. The results showed that, after GABA pre-protection was given to the mice, the lung tissue W/D ratio was significantly decreased, suggesting that GABA effectively alleviated pulmonary edema and prevented protein edema fluid from exuding into lung tissues; thereby ALI symptoms were alleviated.
GABA is decarboxylated by glutamate in the brain via GABA bypass with a flexible structure and the form of zwitterions [12]. GABA not only is the main inhibitory neurotransmitter in the brain, but also plays an important role in the morphogenesis and maturation of various tissues other than the central nervous system. It can exert various physiological functions through its two types of receptors: GABAA and GABAB receptors [12]. GABA-A receptors can form ligand-gated chloride channels, while GABA-B receptors belong to the G protein-coupled receptor family. GABAA receptor agonists include benzodiazepines (tranquilizers), barbiturates and pagoclone, which activate GABAergic pathways by directly binding to GABAA receptors and enhance the effect of GABA on inhibition of synaptic transmission. They have anti-anxiety, anti-epileptic seizures, anti-psychosis and anti-mania effects in the central nervous system [13]. GABAB receptor agonist is used as a muscle relaxant to attenuate pulmonary artery contractions and baclofen (GABAB-agonist) even in pulmonary veins [14]. There are many conflicting reports about which receptor of GABA exerts its anti-injury protective effect in ALI. Some reports showed that propofol, peripheral benzodiazepine receptor ligand Ro5-4864 or anesthetic regimes can reduce ALI by up-regulating the GABA type A receptor [15–17]. However, another report showed that the GABA type A receptor could aggravate the inflammatory response syndrome and oxidative stress in the lungs and play an essential role in LPS-induced ALI [18]. Meanwhile, some reports showed that airway smooth muscle (ASM) expresses a large amount of GABAA and GABAB; GABAB agonists can inhibit ASM contraction, microvascular leakage, etc [19, 20]. Airway epithelium can also express a large amount of GABAA and GABAB, and GABAB agonists can promote epithelial cell proliferation and mucus clearance [21], indicating that activation of the GABAB receptor plays a role against ALI. In the present ALI models, the expression levels of both GABAA and GABAB receptors were enhanced. After exogenous GABA treatment, the expression of receptor GABAA was significantly decreased, and GABAB was significantly enhanced, indicating that the inflammatory response can be inhibited effectively by down-regulating the GABAA receptor and up-regulating the GABAB receptor.
This study is the first to explore the expression of GABA receptors in ALI and confirm that type B receptors play an important protective role against ALI. However, the main problem in this study is that it is not clear whether the doses of GABA and the severity of ALI affect the expression of two types of receptors and the binding of GABA with its receptors. The more specific mechanism of action of GABA on ALI still needs further study.
In conclusion, GABA can improve LPS-induced ALI, inhibit lung tissue edema and inflammatory cell infiltration during ALI, regulate the release of inflammatory mediators, and adjust the balance of pro-inflammatory and anti-inflammatory factors by down-regulating the GABAA receptor and promoting activation of the GABAB receptor. GABA may be a candidate drug for future treatment of ALI.