We live in interesting times, when we finally have the possibility of effective treatment of patients with lipid disorders, and in fact with numerous effective drugs available, it should be rare, at least in theory, to have patients over the lipid targets. But it wasn’t always like that. Thus, we decided to look back on the past and discuss what has had the biggest impact on lipids in the last four decades – which is no coincidence, as on January 20th, 1984, the pivotal trial that confirmed the role of lipid lowering was published in JAMA [1], and all started from there. We would like to emphasize that this is a subjective opinion, and many other experts would have focused on different aspects than those presented here.
Diagnostics: confirming the role of LDL-C in the process of atherosclerosis
Lipid disorders are the second most common metabolic risk factor for atherosclerotic cardiovascular disease (ASCVD) in the world [2]. ASCVD (coronary artery disease, stroke and peripheral artery disease), in turn, is the main cause of premature death in the world [2]. This is confirmed by the data of the World Health Organization (WHO) and Global Burden of Diseases (GDB), which indicate that 18 million people died due to cardiovascular disease (CVD) in 2019, which represents 32% of all deaths in the world. Among deaths related to CVD, 2/3 of them are due to ASCVD, and therefore are potentially avoidable [2, 3].
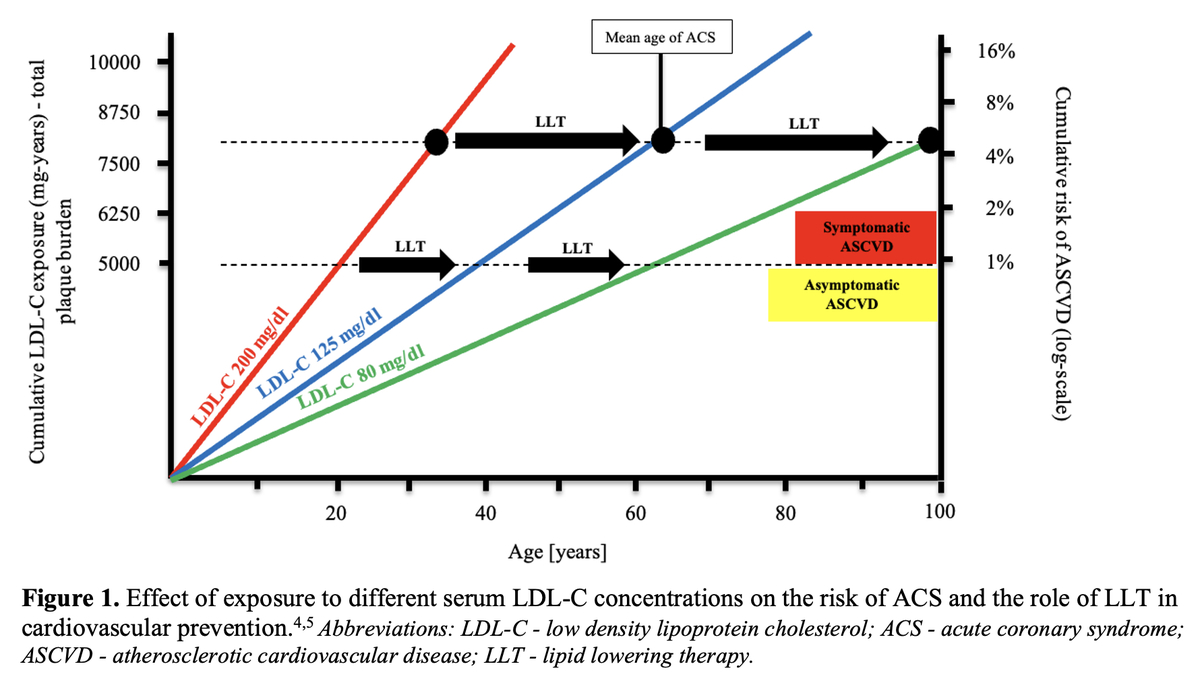
It is very important to emphasize that, in the context of cardiovascular risk, the length of life-long exposure to elevated low-density lipoprotein cholesterol (LDL-C) serum concentration is important (Figure 1) [4, 5].
Figure 1
Effect of exposure to different serum LDL-C concentrations on the risk of ACS and the role of LLT in cardiovascular prevention [4, 5]
LDL-C – low-density lipoprotein cholesterol, ACS – acute coronary syndrome, ASCVD – atherosclerotic cardiovascular disease, LLT – lipid-lowering therapy.
The term “cholesterol-years” represents the cumulative exposure of the arterial wall to LDL-C during the lifetime, and is calculated by multiplying the patient’s age by LDL-C serum concentration. According to the theory, exceeding a certain number of cholesterol-years significantly increases the risk of acute coronary syndrome (ACS). This was confirmed in our recent study of 35,747 patients, which showed that the risk of a first ACS was linearly correlated with the number of cholesterol-years, with a significant risk increase over 6,000 cholesterol-years [6]. The concept of cholesterol-years supports the paradigms of “the lower the better for longer” and “the earlier the better”, as if we started earlier with low LDL-C cholesterol, the cumulative number of cholesterol-years would be reduced and in consequence the risk of the first event would be diminished.
The fundamental importance of lipid disorders in the pathogenesis of ASCVD is indicated by the fact that each reduction of LDL-C by 1% is associated with a reduction in cardiovascular risk by ~1% [7]. Statins and lipid-lowering therapy are among the most effective therapies in cardiology to prevent ASCVD [8]. After 5 years, the risk is reduced by about 20–25%, and after 40 years by as much as 50–55% for each mmol/l of LDL-C [8]. There is no better preventive therapy in cardiology. Therefore, after many years of research, now we know that for the LDL-C level two main paradigms should always be applied: “the lower the better for longer” and “the earlier on LDL-C target, the better” [9, 10].
Diagnostics: residual ASCVD risk factors
Despite effective LDL-C lowering treatment, many patients remain at increased risk of ASCVD, which is associated with the presence of residual lipid risk factors, such as triglycerides ≥ 150 mg/dl (30–40% of statin-treated patients), and lipoprotein (a) (Lp(a)) ≥ 50 mg/dl (about 20% of the general population) [11–13].
It has been shown that elevated triglyceride levels in patients after ACS treated with statins are significantly associated with the risk of cardiovascular events, in both the short- and long-term follow-up [14]. A similar relationship was found in patients in primary prevention who had well-controlled LDL-C levels but had elevated triglycerides (≥ 150 mg/dl) [15].
Elevated Lp(a) levels, regardless of serum LDL-C levels (and other risk factors), are associated with a higher risk of ASCVD [13]. It should be emphasized that an increased level of LDL-C and Lp(a) is a comparably strong risk factor for ASCVD (in the relevant genetic analyses, the risk factor of ASCVD was 2.1 (95% CI: 1.3–3.4) for an increase in LDL-C by every 1 mmol/l and 2.0 (95% CI: 1.6–2.6) for every 1 nmol/l increase in Lp(a)) [16, 17].
So far, there have been no effective pharmacological methods to lower the concentration of triglycerides (with significant cardiovascular benefits) and Lp(a). Recently, the armamentarium of triglyceride-lowering treatment has been supplemented with volanesorsen (a European Medicines Agency-approved therapeutic antisense oligonucleotide (ASO) that targets the messenger RNA for apolipoprotein C-III (apo-CIII)), olezarsen (a currently investigated hepatocyte-targeted modified ASO that reduces apo-CIII plasma levels), and possibly evinacumab (an ANGPTL3 inhibitor, which was approved by the US Food and Drug Administration and EMA in the therapy of homozygous familial hypercholesterolemia) [18, 19]. It also needs to be emphasized that triglycerides and triglyceride-rich lipoproteins can still be effectively reduced with fenofibrate, which, beside its benefits in the reduction of micro- and macrovascular complications, was confirmed to have long-term cardiovascular efficacy in the ACCORDION study [19, 20]. And this approach should not be changed despite the negative results of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study, in which pemafibrate failed to improve cardiovascular outcomes [21]. It seems, however, that study was too short, taking into account the previous experience from trials with fibrates, and, what is even more important, pemafibrate was also the first fibrate that increased the LDL-C by 12%, which definitely affected the primary endpoint result [21]. It is also worth emphasizing that soon we should have very effective drugs to reduce Lp(a) levels by as much as 70–95% (olpasiran, SLN360, LY3819469 and pelacarsen, which are currently undergoing clinical trials) [13, 18, 19, 22]. These therapies might finally help to resolve the problem of lipid-related residual CVD risk.
Therapy: statins – the gold standard in lipidology
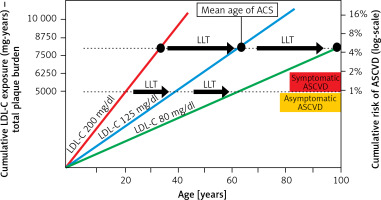
Lipid-lowering treatment plays a crucial role in ASCVD prevention, and statins are the gold standard of lipid-lowering treatment [17]. Everything started with two pivotal studies – the Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) published in 1984 [1] that showed the proof-of-concept of lipid lowering and confirmed that reducing LDL-C levels can diminish the incidence of coronary heart disease (CHD) morbidity and mortality in men at high risk for CHD, and the 4S study [23] that 10 years later confirmed the role of statins in reduction of all-cause mortality. Subsequently, hundreds of studies confirmed these results, and now statins are essential drugs in both primary (Figure 2 A) and secondary (Figure 2 B) prevention of ASCVD, effectively prolonging patients’ lives [24, 25].
Figure 2
The impact of statin use on the prognosis of patients in primary (A) and secondary (B) prevention [24, 25]
CV – cardiovascular, ACS – acute coronary syndrome, CAD – coronary artery disease, MACE – major adverse cardiovascular events.
Despite the class effect, individual statins differ in their lipid-lowering power, so now, in accordance with the Polish guidelines on the diagnosis and treatment of lipid disorders in Poland 2021 and the guidelines of the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) on management in dyslipidemias of 2019, it is recommended to use atorvastatin and rosuvastatin (so-called strong statins) in the maximum doses tolerated by the patient (with the exception of patients with statin intolerance) [22, 26]. Finally, since 2021, there has also been available in many European countries pitavastatin, which reduces LDL-C by 43–47% (4 mg) and seems to have quite unique triglyceride-lowering properties (by as much as 30%) with the best safety profile (the risk of new onset diabetes and myalgia comparable to placebo) [22]. However, due to data being available mostly from Asian countries, these properties need to be further confirmed in studies with European cohorts [27, 28].
In addition to their effectiveness, statins are also characterized by a very good safety profile, surpassing that of most other cardiological drugs. Unfortunately, they do not have good press; statin intolerance is highly overdiagnosed, and the nocebo/drucebo effect is responsible for as much as 70–90% of the adverse effects [29, 30]. The world’s largest meta-analysis, by Bytyçi et al., summarized the global prevalence of statin intolerance (SI). The meta-analysis included 176 studies. The general SI prevalence was only 9.1%, and when diagnosed with the approved definitions it was between 5.9 and 7% [31].
The use of statins is associated with some risk of diabetes (although significantly outweighed by the benefits). Therefore, in patients at increased risk of this disease, experts recommend the use of pitavastatin or the combination therapy of statin and ezetimibe, as well as bempedoic acid, which also does not increase the risk of new onset diabetes [27]. The recent 2022 International Lipid Expert Panel (ILEP) recommendations on statin intolerance (SI) management cover both how to diagnose and treat statin-associated side effects and how to diagnose and overcome the nocebo/drucebo effect [32, 33].
Therapy: combinations of lipid-lowering drugs – fixed-dose combination
Effective non-statin therapy for many years in fact did not exist (besides some negative attempts with CETP inhibitors, niacin, and bile sequestrants), and ezetimibe, which has been available since 2002/2023, was underutilized in most countries, with application in only 1–3% of patients [22]. Only proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors have changed the approach of intensive lipid-lowering combination therapy instead of intensive statin therapy in high to extremely high-risk CVD patients [34, 35]. Alirocumab and evolocumab (monoclonal antibodies) as well as inclisiran (siRNA) have the strongest lipid-lowering effect (↓ LDL-C by 44–65%) [22, 36, 37]. These drugs significantly improve the cardiovascular prognosis of patients with ASCVD, with longer treatment yielding a better effect; however, no outcome data are yet available for inclisiran [25, 38–40]. However, after all those years of investigations, in 2023 a real breakthrough in the prevention of ASCVD is the possibility of using lipid-lowering combination therapies (preferably as a fixed-dose combination – FDC), which allows for LDL-C reduction by even > 85% (Figure 3) [41].
Figure 3
How to be effective with lipid-lowering therapy in ASCVD patients (the size of the LDL-C reduction for some recommended combinations is an assumption and still needs to be confirmed). Reprinted with permission from Banach et al. [41]
*FDC of high-intensity statin therapy and ezetimibe is a preferable option for all ASCVD patients. ASCVD – atherosclerotic cardiovascular disease, LDL-C – low-density lipoprotein cholesterol, ASC – acute coronary syndrome, PAD – peripheral artery disease, HIS – high-intensity statin therapy, FDC – fixed-dose combination, EZE – ezetimibe, BA – bempedoic acid, PCSK9i – proprotein convertase subtilisin/kexin 9 targeted approach therapy (PCSK9 inhibitors + inclisiran).
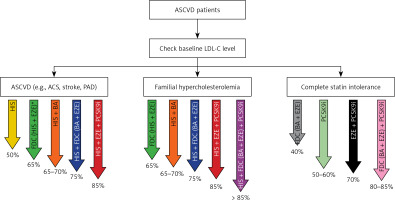
Such a high lipid-lowering potential enables good control of LDL-C in patients at the highest CVD risk and high baseline level of LDL-C, including individuals with familial hypercholesterolemia, statin intolerance and those at very high and extremely high cardiovascular risk, in whom we expect the LDL-C level to be as low as possible, and for which we should always consider upfront lipid-lowering combination therapy [34, 41].
Although we are here focusing on the past, we cannot completely separate this from the present and future perspective. Thus, it is worth emphasizing that bempedoic acid may be a breakthrough in the lipid-lowering treatment of high- and very high-risk patients, including those with statin intolerance [42–44]. The effect of bempedoic acid on cardiovascular risk was evaluated in the recently published CLEAR Outcomes study of 13,970 statin-intolerant high-risk cardiovascular patients. Bempedoic acid 180 mg/day was associated with 13% reduction of the primary endpoint, including a reduced risk of fatal and non-fatal myocardial infarction (HR = 0.77; 95% CI: 0.66–0.91) and the need for coronary revascularization (HR = 0.81; 95% CI: 0.72–0.92) over a 40.6-month follow-up period [42]. Another very beneficial effect of bempedoic acid is the reduction of the risk of de novo diabetes (OR = 0.59; 95% CI: 0.39–0.90), which still requires further examination [43]. FDCs containing bempedoic acid and ezetimibe have the potential to reduce LDL-C by over 40% [44]. The latest ILEP guidelines summarize the place and role of bempedoic acid in the treatment of high CVD risk patients [44].
Conclusions
Obviously, these diagnostic and therapeutic achievements in the last four decades have been briefly presented from the authors’ very subjective point of view. These developments directly influence what we are seeing in the area of lipid disorders now and what we will be seeing soon, and we also briefly alluded to this in our opinion. However, irrespectively of what we have presented here, one thing deserves to be emphasized: nowadays lipid disorders are among the most intensively investigated medical fields, with the largest number of ongoing trials, together with palliative medicine and oncology. Finally, we have effective diagnostic methods and drugs that allow us to be effective and prevent the first and recurrent cardiovascular events. We now only need to put our strongest emphasis on education – both the ongoing one of physicians and healthcare employees and that of patients – because we need to have well-trained physicians to diagnose and treat lipid disorders early and effectively, and especially well-educated and convinced patients (which is indeed a great challenge in these times of science denialism and anti-statin movements) to be adherent and compliant and in consequence optimally protected against CVD [45, 46].
One should treat this opinion paper also from the point of view of forthcoming developments in the field of lipidology. In our opinion, two areas of lipid disorders that will dramatically change soon are the one associated with diagnosis and especially effective therapy of severe hypertriglyceridemia with the new drugs that target Apo-CIII or angiopoietin-like protein 3 (ANGPLT3) (evinacumab, volanesorsen, olezarsen) [47]. Considering another negative trial with pemafibrate, these drugs will be of great importance; however, on the other hand, we cannot definitely refer the PROMINENT results to fenofibrate (and bezafibrate) [21]. Hopefully we will also be able to solve all the doubts associated with administration of omega-3 acids, with the suitable preparations, doses, effects on CVD outcomes, and safety – here the real world evidence should bring us suitable knowledge and answer all important questions [22]. Finally, just around the corner, we have finally effective and targeted Lp(a) drugs with the potential to reduce Lp(a) by > 90% [13]. But here we need to take care to have Lp(a) measured commonly, as we have already done for non-HDL-C. On the other hand, I strongly believe that for Lp(a), we still know less than we think, and that is why the future of lipidology seems to be so fascinating…
Conflict of interest
Maciej Banach: speakers bureau – Amgen, Daiichi Sankyo, Kogen, KRKA, Polpharma, Novartis, Novo-Nordisk, Pfizer, Sanofi-Aventis, Teva, Viatris, Zentiva; consultant to Adamed, Amgen, Daichii Sankyo, Esperion, Freia Pharmaceuticals, NewAmsterdam, Novartis, Novo-Nordisk, Polfarmex, Sanofi-Aventis; grants from Amgen, Daiichi Sankyo, Mylan/Viatris, Sanofi and Valeant; CMDO at the Longevity Group, CMO at Nomi Biotech Corporation; Stanisław Surma – honoraria from Novartis/Sandoz.