Introduction
Heart failure (HF) is still a major cause of morbidity and mortality all over the world [1, 2]. Despite the effectiveness of prevention, morbidity is still increasing [3]. In the USA, the prevalence of chronic heart failure (CHF) is over 5.7 million, with 670,000 new cases yearly and a cost of about USD 32 billion annually in treatment expenditures and lost productivity [4]. Farré et al. found that 8.8% of HF patients had an HF hospitalisation at 1-year follow-up; however, about 30% had an all-cause hospitalization [5]. CHF hospitalisation rates are about 1% to 2% of all hospitalisations yearly and are the leading cause of hospital stay in patients over 65 years of age [6].
Advanced device-based therapies have prolonged survival in HF patients; however, optimisation of pharmacological treatment remains the principal method of management. Currently, 4 types of HF are defined in actual guidelines: HF with reduced LVEF (< 40%; HFrEF), HF with mid-range LVEF (40% to 49%; HFmrEF), HF with preserved LVEF (≥ 50%; HFpEF), and HF with improved HF [1, 2]. Sacubitril/valsartan (S/V, formerly known as LCZ696) represents a novel form of pharmacotherapy that acts by enhancing the natriuretic peptide system via inhibition of neprilysin and by suppressing the renin-angiotensin-aldosterone system (RAAS) via AT1 receptor blockade, thereby producing more effective neurohormonal modulation than can be achieved with RAAS inhibition alone [7]. Inhibition of neprilysin increases the levels of natriuretic peptides (NP) and decreases vasoconstriction, abnormal growth, sodium retention, and remodeling [8]. The addition of an ARB to the neprilysin inhibitor is necessary to prevent activation of RAAS.
The Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor (ARNI) with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial demonstrated improved morbidity and mortality with the combination of neprilysin inhibitor/angiotensin receptor blocker S/V [9, 10]. In comparison to enalapril, sacubitril/valsartan reduced the occurrence of the primary outcome (cardiovascular death or hospitalisation for HF) by 20% and delivered a 16% reduction in all-cause mortality [11]. The 2021 ESC guidelines still recommend the use of ARNI as a replacement for angiotensin-converting enzyme inhibitors (ACEI) in suitable HFrEF patients who remain symptomatic on ACEI, β-blocker, and mineralocorticoid receptor antagonist (MRA) therapies; however, an ARNI may also be considered as a first-line therapy instead of an ACEI. According to these recommendations, treatment with an ARNI may be considered in patients with HFmrEF but with no recommendation for HFpEF [1]. The 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure recommends switching patients who are on an ACEI or angiotensin receptor blocker (ARB) to an ARNI if they have chronic symptomatic HFrEF with NYHA class II or III symptoms, because of improvement in morbidity and mortality. Sacubitril/valsartan is recommended also as a de novo treatment in hospitalized patients with acute HF before discharge. In selected patients with HFmrEF and HFpEF ARNI may be considered, to decrease hospitalizations, particularly among patients with LVEF on the lower end of this spectrum [2]. The benefits and harms of sacubitril/valsartan in all chronic HF types have not been formally evaluated in a systematic review.
The aim of this systematic review was to assess the benefits and harms of first-in-class ARNI S/V as compared to ACEI or ARB in HF patients.
Material and methods
Study searches
We identified randomized controlled trials (RCTs) through systematic searches of the following bibliographic databases on 2 August 2021: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Embase Ovid, Web of Science Core Collection, and Scopus. We also conducted searches in ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) Search Portal (www.apps.who.int/trialsearch) for ongoing or unpublished trials. Search strategies are available in the supplement materials.
Study selection
We included published and unpublished parallelarm, phase 2, 3, and 4 RCTs comparing S/V (LCZ696) with ACEI or ARBs. Adult patients (18 years of age or older) with a diagnosis of acute or chronic HF regardless of the value of the ejection fraction were eligible. The exclusion criteria in relevant sacubitril/valsartan RCTs were also exclusion criteria for our review: known history of angio-oedema [12]; requirement of treatment with both ACEIs and ARBs [12, 13]; serum potassium greater than 5.2 mmol/l [12], eGFR < 15 ml/min/1.73 m² (www.ema.europa.eu/en/documents/product-information/entresto-epar-product-information_en.pdf); and hypersensitivity or allergy to any study drugs, drugs of similar chemical classes, and known contraindications or suspected contraindications to study drugs [12, 13].
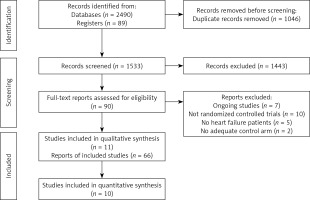
Two review authors (AMB-D and AVH) independently screened titles and abstracts for inclusion of all the potential studies and coded them as ‘retrieve’ (eligible or potentially eligible/unclear) or ‘do not retrieve’. If there were any disagreements, we consulted a third author (MB). We retrieved full-texts, and the same 2 authors (AMB-D and AVH) independently screened them; the authors identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third investigator (MB). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of analysis in the review. We recorded the selection process in sufficient detail to complete a PRISMA 2020 flow diagram [14].
Outcomes
We used RCT definitions for all outcomes. We reported the longest available follow-up data and noted whether or not it was an intention-to-treat (ITT) population. Primary outcomes were heart failure hospitalisations (assessed as participants with at least one event and time to first event during follow-up) and cardiovascular (CV) mortality. Secondary outcomes were all-cause mortality, all-cause hospitalisation (assessed as participants with at least one event and time to first event during follow-up), kidney function measured with eGFR, myocardial dysfunction, measured by several methods such as increased BNP or NT-proBNP levels (e.g. > 100 pg/ml or > 400 pg/ml, respectively), decreased LVEF (e.g. < 25%), increased early mitral filling velocity (E)/early diastolic mitral annular velocity (E’) ratio (E/E’) (e.g. > 15), systolic blood pressure, diastolic blood pressure, quality of life, and serious adverse events (SAE) defined according to the FDA (www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event). Adverse events of interest included worsening renal function (elevated creatinine ≥ 2 mg/dl), hyperkalaemia (serum K+ ≥ 5.5 mmol/l), symptomatic hypotension (SBP < 100 mm Hg) and angio-oedema.
Data extraction
Three investigators (NS, JM, VP) independently extracted information from RCTs. Discrepancies were resolved by discussion and, if required, with consultation with 2 other investigators (AMB-D and AVH). Extracted information included RCT acronym, first author, year of publication, RCT phase, sample size, type of HF patient, follow-up time, S/V dose and duration, type of comparator, and primary and secondary outcomes per arm.
Risk of bias assessment
Risk of bias (RoB) of individual RCTs was performed with the Cochrane risk of bias tool [15]. Each study was evaluated independently evaluated by 2 authors (NS, JM), and disagreements were resolved by discussion with a third investigator (AVH). Eight questions about randomization methods, allocation concealment, blinding of patients, personnel and outcome assessors, incomplete outcome data, selective reporting of outcomes, and other biases were responded as having high, unclear, or low RoB. Then, every RCT was labelled as having high RoB when randomization or blinding questions were at high RoB, and as having unclear RoB when one or more questions were at unclear RoB and none at high RoB.
Statistical analysis
We narratively described skewed data reported as medians and interquartile ranges.
We used random-effects models for our meta-analyses and the DerSimonian and Laird method was used for calculating between-study variance (tau²) [16]. Effect of S/V effects on outcomes was described with risk ratio (RR) or hazard ratio (HR) with 95% confidence interval (95% CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) with 95% CI for continuous outcomes. We preferred HR to RR when follow-up time for RCTs was longer than 6 months. When continuous outcomes were measured using different scales, we used SMD; otherwise, we used MD. When not provided by authors, we calculated the standard deviation (SD) of a MD between final and baseline using the formula: SQRT((SD1)2 + (SD2)2 – (2*0.75*SD1*SD2)), where SD1 is the final SD, SD2 is the SD at baseline, and 0.75 is the correlation coefficient r of the continuous outcome at the 2 time points (https://onlinestatbook.com/2/tests_of_means/correlated.html).
Our primary comparison of interest was S/V vs. ACEI or ARBs. Secondarily, we compared sacubitril/valsartan vs. ACEI, and sacubitril/valsartan (LCZ696) vs. ARBs. Presence of statistical heterogeneity among effects was defined as p < 0.10 in the χ2 test [17]. We used the I² statistic to measure the amount of heterogeneity per outcome; substantial heterogeneity was defined as I² > 60% [17]. We also considered very substantial heterogeneity with I2 > 90%. There was uncertainty in the value of I² when there was only a small number of studies. We used RevMan 5.4 for all analyses (Review Manager [Computer program] Version 5.4. The Cochrane Collaboration, 2020).
Two review authors (VP, AMB-D) independently made judgements about GRADE certainty of evidence (CoE) per outcome (www.gradeworkinggroup.org), with disagreements resolved by discussion with a third author (AVH). We created Summary of findings (SoF) tables for each of the 3 comparisons using www.gradepro.org (GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022).
Results
Study selection
We identified 2579 records in our searches, 1533 records after de-duplication, and assessed 90 full-text articles for inclusion (Figure 1). We finally selected 66 articles, representing 11 unique RCTs (n = 18766) (EVALUATE-HF 2019 [18]; PARADIGM-HF 2014 [8]; PARAGON-HF 2019 [19]; PARAMOUNT 2012 [9]; PIONEER-HF 2019 [20]; PRIME 2019 [21]; AWAKE-HF 2021 [22]; Tumasyan 2019 [23]; Fan 2020 [24]; OUTSTEP-HF 2021 [25]; and PARALLAX 2021 [26]). The characteristics of included studies are presented in Table I.
Table I
Study characteristics of included RCTs
| Study, year [reference] | Type of RCT | No. | Type of HF | Inclusion criteria | Study comparison | Primary endpoints | Follow-up time |
|---|---|---|---|---|---|---|---|
| AWAKE-HF 2021 [22] | Multicentre, randomised, double-blind clinical trial | 147 | HFrEF | NYHA class II or III | Sacubitril/valsartan vs. enalapril | Ratio of mean activity counts collected during the most active 30 min of the subject’s day between week 8 and baseline | 16 weeks |
| EVALUATE-HF 2019 [18] | Randomised, double-blind clinical trial | 464 | HFrEF | Hypertension and SBP > 105 mm Hg on antihypertensive medication. SBP ≥ 140 mm Hg not on antihypertensive medication. NYHA class I–III heart failure and with reduced ejection fraction ≤ 40% | Sacubitril-valsartan vs. enalapril | Change from baseline in aortic characteristic impedance at week 12 | 12 weeks |
| Fan 2020 [24] | Randomised clinical trial | 120 | HF | HF | Sacubitril/valsartan vs. valsartan | Adverse drug reactions, stroke volume (SV), left ventricular end-diastolic diameter LVEDD), LVEF, 6-minute walk test 6MWT), N-terminal brain natriuretic peptide-NT-BNP), and cardiac troponin I cTnI | 12 weeks |
| OUTSTEP-HF 2021 [25] | Randomised, double-blind, actively controlled | 621 | HFrEF | NYHA class II–IV with LVEF ≤ 40% and NT-proBNP ≥ 300 pg/ml or BNP ≥ 100 pg/ml | Sacubitril/valsartan vs. enalapril | Change from baseline in exercise capacity and mean daily non-sedentary daytime activity | 14 weeks |
| PARADIGM-HF 2014 [8] | Multicentre, randomised, double-blind, parallel group, active-controlled | 8442 | HFrEF | Outpatients, NYHA class II–IV and LVEF ≤ 35% and elevated BNP | Sacubitril/valsartan vs. enalapril | Occurrence of the composite endpoint, which is defined as either cardiovascular death or HF hospitalization | 51 months |
| PARAGON-HF 2019 [19] | Multicenter, randomised, double-blind, parallel Group, active-controlled | 4822 | HFmrEF and HFpEF | LLVEF ≥ 45% and symptom(s) of HF requiring treatment with diuretic(s) at least 30 days prior to study entry structural heart disease, elevated NT-proBNP | Sacubitril/valsartan vs. valsartan | Cumulative number of primary composite events of cardiovascular death and total (first and recurrent) HF hospitalizations during follow-up (57 months) | 57 months |
| PARALLAX 2021 [26] | Randomised, double-blind, multi-centre, parallel Group, active controlled | 2569 | HFmrEF and HFpEF | LVEF > 40% within 6 months prior to study entry or during the screening HF symptoms requiring treatment with diuretics for at least 30 days prior to study entry, NYHA class II–IV, structural heart disease, NT-proBNP > 220 pg/ml for patients with no atrial fibrillation/atrial flutter or > 600 pg/ml for patients with AF, KCCQ clinical summary score < 75, patients on ACEi or ARB therapy must have a history of hypertension | Sacubitril/valsartan vs. enalapril or valsartan | Change from baseline NT-proBNP at week 12 Change from baseline in 6-minute walk distance at week 24 | 24 weeks |
| PARAMOUNT 2012 [9] | Randomised, double-blind, multi-centre, parallel Group, active controlled | 307 | HFmrEF and HFpEF | NYHA II–IV, LVEF ≥ 45%; NT-proBNP > 500 pg/ml | Sacubitril/valsartan vs. valsartan | Change from baseline in NT-proBNP | 26 weeks |
| PIONEER-HF 2019 [20] | Multicentre, randomised, double-blind, double dummy, parallel group, active-controlled | 887 | HFrEF | Currently hospitalized for ADHF who meet the following definition of stable status: SBP ≥ 100 mm Hg for the preceding 6 h prior to randomization; no symptomatic hypotension. LVEF ≤ 40% within the past 6 months (including current hospitalization). Elevated NT-proBNP ≥ 1600 pg/ml OR BNP ≥ 400 pg/ml during current hospitalization | Sacubitril/valsartan vs. enalapril | NT-proBNP values and time-averaged change from baseline | 8 weeks |
| PRIME 2019 [21] | Multicentre, randomised, double-blind, active-controlled | 118 | HFrEF and HFmrEF | Outpatients with NYHA class II or III | Sacubitril/valsartan vs. valsartan | Change of effective regurgitant orifice area (EROA) of functional mitral regurgitation from baseline to 12 months follow-up | 12 months |
| Tumasyan 2019 [23] | Randomised, phase 2 clinical trial | 334 | HFrEF, HFmrEF, HFpEF | Sinus rhythm and NYHA III chronic HF | Sacubitril/valsartan vs. ramipril vs. valsartan | One-year mortality and hospitalizations | 12 months |
Trial characteristics
Five RCTs had ACEIs as controls, 5 RCTs had ARBs as controls, and one RCT had ACEI or ARB as control. Follow-up times ranged between 2 and 48 months. All studies, except Fan 2020 [24], provided outcome data for meta-analyses (n = 18646). Several secondary references of 6 RCTs (EVALUATE-HF 2019 [18]; PARADIGM-HF 2014 [8]; PARAGON-HF 2019 [19]; PARAMOUNT 2012 [9]; PIONEER-HF 2019 [20]; PRIME 2019 [21]) reported subgroup effects by age, LVEF, chronic kidney disease, use of mineralocorticoid receptor antagonists, diuretic use, β-blocker target dose < 50%, and sex.
Included RCTs were conducted in outpatient chronic HF patients, except the PIONEER-HF RCT, which was performed in hospitalised acute HF patients [20]; Fan 2020 did not specify the type of HF patient [24]. RCTs were performed in patients with reduced EF, except the PARAGON-HF, PARAMOUNT, and PARALLAX RCTs performed in preserved EF patients [9, 19, 26]; one RCT evaluated HF patients with preserved, mid-range, and reduced EF [23]. In the EVALUATE-HF and PARAMOUNT RCTs there were HF patients in I, II, and III NYHA classes [9, 18], in PIONEER-HF, PARADIGM and PARAGON-HF RCTs in all 4 NYHA classes [8, 19, 20], in PRIME and AWAKE-HF RCTs in II and III NYHA classes [21, 22], in the Tumasyan et al. RCT only in III NYHA class [23], and in OUTSTEP-HF and PARALLAX in II, III and IV NYHA classes [25, 26].
Among the included RCTs, patients’ mean ages ranged between 61 and 73 years, and the proportion of males ranged between 48% and 79%. There were 34% diabetic patients in the PARADIGM-HF study, 43% in PARAGON-HF, 38% in PARAMOUNT, 19% in PIONEER-HF, 31% in PRIME, and 34% in OUTSTEP-HF [8, 9, 19, 20, 21, 25]. Decline in renal function was described across RCTs as a composite of death from renal failure, end-stage renal disease (ESRD) or decrease > 50% in eGFR from baseline, and 2 RCTs comparing S/V vs. ARB reported decline in eGFR at follow-up [9, 19].
Effects of sacubitril/valsartan vs. ACEI or ARB
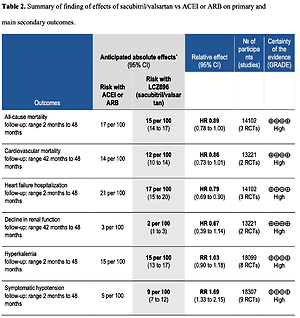
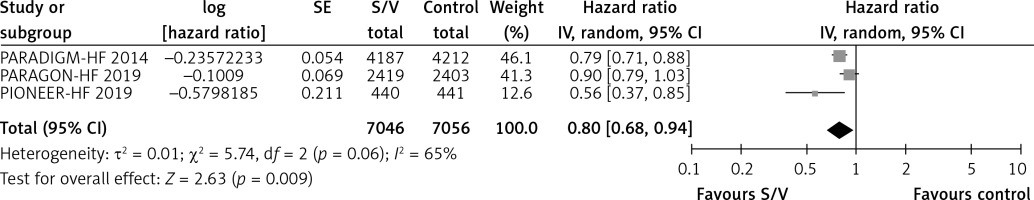
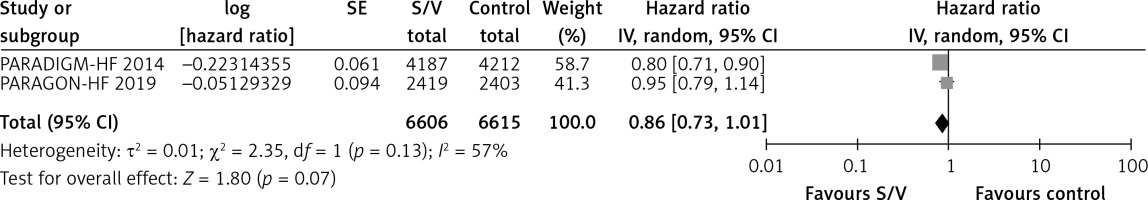
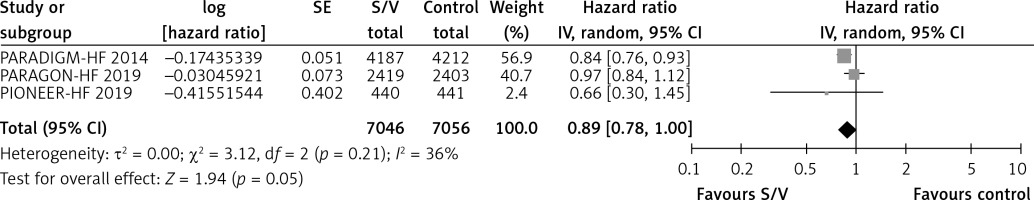
Table II shows the effects of S/V vs. ACEI or ARB control on primary and main secondary outcomes. S/V reduced the hazards of heart failure hospitalisation by 20% (HR = 0.80, 95% CI: 0.68–0.94; 3 RCTs; n = 14102; I2 = 65%; high CoE, Figure 2), CV mortality by 14% (HR = 0.86, 95% CI: 0.73–1.01; 2 RCTs; n = 13221; I2 = 57%; high CoE, Figure 3), and all-cause mortality by 11% (HR = 0.89, 95% CI: 0.78–1.00; 3 RCTs; n = 14102; I2 = 36%; high CoE, Figure 4). The PARALLAX RCT [26] comparing S/V vs. ACEI or ARB found an increase of all-cause mortality (LCZ696 23/1280 vs. ACEI or ARB 17/1284, RR = 1.36, 95% CI: 0.73–2.53; n = 2564); this RCT posted results on clinicaltrials.gov only.
Table II
Summary of finding of effects of sacubitril/valsartan vs. ACEI or ARB on primary and main secondary outcomes
S/V reduced the hazard of decline in renal function by 33% (HR = 0.67, 95% CI: 0.39–1.14; 2 RCTs; n = 13221; I2 = 78%; high CoE, Supplementary Figure S1), and there was a slight increase in eGFR from baseline vs. control (MD 1.26 ml/min/1.73 m2, 95% CI: –0.92 to 3.44; 2 RCTs; n = 4938; I2 = 40%, Supplementary Figure S2). S/V slightly reduced LVEF (MD = –0.13%, 95% CI: –1.24% to 0.99%; 3 RCTs; n = 703, I2 = 0%, Supplementary Figure S3) and the E/E’ ratio (MD = –1.40, 95% CI: –2.72 to –0.08; 3 RCTs; n = 621; I2 = 61%, Supplementary Figure S4). Other secondary outcomes were reduced by S/V: NT-proBNP (SMD: –0.34, 95% CI: –0.52 to –0.16; 3 RCTs; n = 1371; I2 = 62%; Supplementary Figure S5), hs-TNT (ratio of differences = 0.84, 95% CI: 0.79–0.88; 2 RCTs; n = 1293, I2 = 0%, Supplementary Figure S6), SBP (MD: –4.4 mmHg, 95% CI: –6.59 to –2.22; 4 RCTs; n = 9225; I2 = 68%, Supplementary Figure S7), and DBP (MD: –2.83 mm Hg, 95% CI: –4.02 to –1.65; 3 RCTs; n = 826, I2 = 0%, Supplementary Figure S8). The PARALLAX RCT [26] reported a reduction in the ratio of change of geometric mean of NT-proBNP levels between S/V and ACEI or ARB (ratio = 0.84, 95% CI: 0.80–0.88; n = 2417). S/V slightly improved KCCQ-QoL scores (MD = 1.36 points, 95% CI: 0.49–2.24; 6 RCTs; n = 16,404; I2 = 54%, Supplementary Figure S9).
Among the AEs, worsening serum creatinine or eGFR was reduced by 7% with S/V (RR = 0.93, 95% CI: 0.74–1.17; 8 RCTs; n = 18,168; I2 = 55%, Supplementary Figure S10), and symptomatic hypotension was increased by 69% (RR = 1.69, 95% CI: 1.33–2.15; 9 RCTs; n = 18,307; I2 = 65%; high CoE, Supplementary Figure S11). Other AEs were similar between S/V and controls: hyperkalaemia (RR = 1.03, 95% CI: 0.90–1.18; 8 RCTs; n = 18,099; I2 = 47%; high CoE, Supplementary Figure S12) and angioedema (RR = 1.44, 95% CI: 0.62–3.32; 7 RCTs; n = 18,050; I2 = 31%, Supplementary Figure S13).
Effects of sacubitril/valsartan vs. ACEI alone
Table III and Supplementary Figures S14 to S23 show the effects of S/V vs. ACEI control on clinical outcomes and adverse events. S/V reduced the hazard of heart failure hospitalisation by 29% HR = 0.71, 95% CI: 0.52–0.97; 2 RCTs; n = 9280; I2 = 60%; high CoE, Supplementary Figure S14), cardiovascular mortality by 20% (HR = 0.80, 95% CI: 0.71–0.90; 1 RCT; n = 8399) [8], and all-cause mortality by 16% (HR = 0.84, 95% CI: 0.76–0.92; 2 RCTs; n = 9280; high CoE, Supplementary Figure S15). One-year all-cause mortality was not different between S/V and control in Tumasyan 2019 RCT reported as abstract only (S/V 25/80 vs. ACEI 33/87, RR = 0.82, 95% CI: 0.54–1.26; n = 167) [23]. OUTSTEP-HF RCT comparing S/V vs. ACEI found a non-significant reduction of all-cause mortality (S/V 1/309 vs. ACEI 4/310, RR = 0.25, 95% CI: 0.03–2.23; n = 619) [25]. S/V reduced all-cause hospitalisations by 25% in comparison to ACEI control in the Tumasyan RCT (RR = 0.75, 95% CI: 0.56–0.99; n = 167) [23]. S/V reduced the hazard of decline of renal function (end-stage renal disease, death from renal failure, or eGFR reduction of 50% or more) by 14% (HR = 0.86, 95% CI: 0.65–1.14; 1 RCT; n = 8399) [8]. S/V did not increase LVEF (MD = 0%, 95% CI: –2.09% to 2.09%; 1 RCT, n = 394) [18] or reduce NT-proBNP (MD = –479 pg/ml, 95% CI: –1082.2 to 124.2; 2 RCTs; n = 1140; I2 = 95%, Supplementary Figure S16). Other intermediate outcomes were reduced by S/V : E/E’ ratio (MD = –1.9, 95% CI: –2.97 to –0.83; 1 RCT, n = 339) [18] and hs-TNT (ratio of differences = 0.84, 95% CI: 0.79–0.88; 2 RCTs; n = 1293, I2 = 0%, Supplementary Figure S17). SBP (MD = –3.95 mm Hg, 95% CI: –6.93 to –0.97; 2 RCTs; n = 8820; I2 = 80%, Supplementary Figure S18) and DBP (MD = –2.6 mm Hg, 95% CI: –4.11 to –1.09; 1 RCT; n = 421) [18] were also reduced by S/V. KCCQ-QoL scores were slightly improved by S/V (MD = 2.70 points, 95% CI: –0.38 to 5.79; 3 RCTs; n = 8957; I2 = 67%, Supplementary Figure S19).
Table III
Summary of finding of effects of sacubitril/valsartan vs. ACEI on primary and main secondary outcomes
Among the AEs, worsening serum creatinine or eGFR was reduced by 20% with S/V (RR = 0.80, 95% CI: 0.68–0.96; 4 RCTs; n = 1036, I2 = 0%; Supplementary Figure S20), and symptomatic hypotension was increased by 71% (RR = 1.71, 95% CI: 1.25–2.33; 5 RCTs; n = 10,502; I2 = 41%; high CoE, Supplementary Figure S21). Other AEs were similar between S/V and ACEI: hyperkalaemia (RR = 1.17, 95% CI: 0.88–1.55; 4 RCTs; n = 10,363; I2 = 60%; high CoE, Supplementary Figure S22) and angioedema (RR = 0.88, 95% CI: 0.21–3.58; 4 RCTs; n = 10,363; I2 = 47%, Supplementary Figure S23).
Effects of sacubitril/valsartan vs. ARB alone
Table IV and Supplementary Figures S24 to S33 show the effects of S/V vs. ARB control on clinical outcomes and adverse events. S/V reduced the hazard of heart failure hospitalisation by 10% (HR = 0.90, 95% CI: 0.79–1.03; 1 RCT; n = 4822) [19], cardiovascular mortality by 5% (HR = 0.95, 95% CI: 0.79–1.14; 1 RCT; n = 4822) [19], and all-cause mortality by 3% (HR = 0.97, 95% CI: 0.84–1.12; 1 RCT; n = 4822) [19]. One-year all-cause mortality was not different between S/V and ARB in Tumasyan RCT [23] reported as abstract only (S/V 25/80 vs. ARB 34/83, RR = 0.76, 95% CI: 0.50–1.16; n = 163). S/V reduced all-caused hospitalisation by 29% in comparison to ARB (RR = 0.71, 95% CI: 0.54–0.94; 1 RCT; n = 163) in Tumasyan RCT [23]. S/V reduced the hazard of decline in renal function by 50% (HR = 0.50, 95% CI: 0.33–0.76; 1 RCT; n = 4822; high CoE) [19], and there was a slight increase in eGFR from baseline with S/V vs. ARB (MD = 1.26 ml/min/1.73 m2, 95% CI: –0.92 to 3.44; 2 RCTs; n = 4938; I2 = 40%, Supplementary Figure S24). Echocardiographic parameters such as LVEF were slightly decreased (MD = –0.18%, 95% CI: –1.49% to 1.14%; 2 RCTs; n = 309, I2 = 0%, Supplementary Figure S25), and the E/E’ ratio was decreased (MD = –1.22, 95% CI: –3.43 to 0.99; 2 RCTs; n = 282; I2 = 67%, Supplementary Figure S26) by S/V. However, NT-proBNP (Geometric MD [GMD] = –52 pg/ml, 95% CI: –172.01 to –68.01; and ratio of change of GMs = 0.77, 95% CI: 0.64–0.92; 1 RCT; n = 231) [9] was reduced by S/V. Also, blood pressure levels were reduced by S/V: SBP (MD = –5.42 mm Hg, 95% CI: –8.13 to –2.71; 2 RCTs; n = 405, I2 = 0%, Supplementary Figure S27) and DBP (MD = –3.21 mm Hg, 95% CI: –5.14 to –1.29; 2 RCTs; n = 405, I2 = 0%, Supplementary Figure S28). S/V slightly improved KCCQ-QoL scores (MD = 1.0 point, 95% CI: 0.98–1.02; 2 RCTs; n = 5030, I2 = 0%, Supplementary Figure S29).
Table IV
Summary of finding of effects of sacubitril/valsartan vs. ARB on primary and main secondary outcomes
Among the AEs, worsening serum creatinine or eGFR was reduced by 14% with S/V (RR = 0.86, 95% CI: 0.66–1.12; 3 RCTs; n = 5241; I2 = 0%, Supplementary Figure S30), and symptomatic hypotension was increased by 43% (RR = 1.43, 95% CI: 1.24–1.65; 3 RCTs; n = 5241; I2 = 0%, Supplementary Figure S31). Hyperkalaemia was similar between S/V and ARBs (RR = 1.02, 95% CI: 0.65–1.58; 3 RCTs; n = 5172; I2 = 44%; Supplementary Figure S32), and angioedema risk was increased with S/V vs. ARBs (RR = 3.43, 95% CI: 1.20–9.78; 2 RCTs; n = 5123, I2 = 0%, Supplementary Figure S33).
Discussion
Main findings
In comparison to ACEI or ARB together, we found that S/V reduced the risk of HF hospitalisation by 20%, the risk of CV mortality by 14%, and the risk of all-cause mortality by 11%. S/V slightly improved quality of life assessed with KCCQ-QoL scores. S/V was associated with small reductions of E/E’, NT-proBNP, and hs-TNT, but without an increase of LVEF levels. SBP and DBP were reduced with S/V, with 69% increased risk of symptomatic hypotension. S/V reduced the hazard of decline of renal function by 33%; hyperkalaemia and angioedema events were similar between S/V and controls.
When comparing S/V separately to either ACEI or ARB, we found reductions of the risk of HF hospitalisation by 29% in 2 studies, CV mortality by 20% in one study, and all-cause mortality by 16% in 2 studies vs. ACEI, as well as risk reductions of HF hospitalisation by 10%, CV mortality by 5%, and all-cause mortality by 3% in one trial vs. ARB. S/V reduced SBP and DBP, but it was associated with more episodes of symptomatic hypotension compared to either ACEI or ARB. S/V slightly improved KCCQ-QoL scores for both comparisons. S/V reduced the E/E’ ratio and hs-TNT compared to ACEI, and NT-proBNP compared to ARB. There were reductions in the risk of decline in renal function: 14% vs. ACEI and 50% vs. ARB, and there also was a slight increase in eGFR levels vs. ARB. Hyperkalaemia, however, was similar between S/V and either ARB or ACEI. Angioedema risk was significantly increased with S/V vs. ARB only.
What is known about the research question?
A previous meta-analysis by Wang et al. compared the effects of S/V versus ACEI or ARB on cardiac reverse remodeling (CRR) [27]. Twenty randomized and non-randomised studies enrolling 10,175 patients were included. The primary study outcomes were changes in functional capacity (NYHA functional class, 6-minute walking distance [6MWD]), CRR indices (LVEF and other parameters of left ventricle function such as end-systolic volume [ESV], end-diastolic volume [EDV], end-systolic diameter [ESD], end-diastolic diameter [EDD], left ventricular mass index [LVMI], and left atrial volume [LAV]), and biomarkers (NT-proBNP, sST2). S/V significantly improved the functional capacity in patients with HFrEF, including increasing NYHA class and 6MWD. The study also showed significant improvements in ventricular EF, diameter, and volume compared with ACEIs or ARBs in HFrEF patients, but with only limited changes in LVMI and LAV in HFpEF patients. In the study by Wang 2019 [27], S/V reduced sST2 in HFrEF but not in HFpEF patients. In contrast to Wang 2019, we did not find an increase in LVEF. In both meta-analyses, S/V therapy reduced NT-proBNP when compared with ACEI or ARB.
Solomon et al. performed a meta-analysis using data from 3 RCTs of HFrEF that evaluated combined neprilysin/RAS inhibition vs. RAS inhibition alone on clinical outcomes: IMPRESS (n = 573), OVERTURE (n = 5770), and PARADIGM-HF (n = 8399) [28]. In the IMPRESS and OVERTURE RCTs, the study drug was omapatrilat, which is not currently available. However, the hazard of the composite outcome of all-cause mortality or heart failure hospitalisation was reduced in patients receiving combined neprilysin/RAS inhibition, with a pooled HR of 0.86 (95% CI: 0.76–0.97, p = 0.013). For all-cause mortality, there was also a significant reduction (HR = 0.88, 95% CI: 0.80–0.98, p = 0.021). In our study, we found a reduction in the risk of all-cause mortality and HF hospitalisation when compared to ACEI and ARB together, and a larger reduction of the risk of HF hospitalization, CV mortality, and all-cause mortality compared to ACEI alone. The lack of effect in analyses vs. ARB alone was due to the inclusion of HFpEF subjects from the PARAGON-HF RCT [9]. Similar to our observations, combined neprilysin/RAS inhibition compared with ACE inhibition was associated with more symptomatic hypotension, but lower decline of renal function.
One of the most important issues that can influence the outcomes of drug therapy is safety. In our meta-analysis, we assessed individual adverse events between S/V and controls based on up to 9 RCTs in patients with HF. S/V reduced the risk of worsening serum creatinine or eGFR but increased the risk of symptomatic hypotension compared to ACEI or ARB. Other adverse events like hyperkalaemia and angioedema were similar between S/V and ACEI or ARB and ACEI alone, but there were more events of angioedema in comparison with ARB alone. A meta-analysis by Li et al. of 6 RCTs with 11,821 subjects with HF or hypertension showed that S/V significantly decreased the risk of serious adverse events and death compared with ACEI or ARB [29]. S/V also significantly decreased the risk of discontinuation of treatment for any adverse events compared with ACEI or ARB or a placebo. Li et al. also found that S/V significantly increased the risk of angioedema, which is similar to our findings in comparison to ARB only, but it is contrary to our findings when compared and ACEI or ARB together and ACEI alone. Li et al. also found a decreased risk of renal dysfunction. Finally, Li et al. found no difference for hypotension or hyperkalaemia between the S/V and ACEI or ARB together. The authors concluded that S/V was associated with fewer adverse events than a placebo and ACEI or ARB. These observations are generally in agreement with our study.
Zhang et al. [30] performed a meta-analysis of RCTs assessing and comparing the effect and adverse events of S/V, valsartan, and enalapril in patients with HF. The authors included 6 studies involving 14,959 patients (EVALUATE-HF 2019; PARADIGM-HF 2014; PARAGON-HF 2019; PARAMOUNT 2012; PIONEER-HF 2019; PRIME 2019); we included all of them in our review. Compared with ACEI or ARB, S/V reduced all-cause mortality and cardiovascular mortality in patients with HFrEF in 3 trials (PARADIGM-HF 2014; PIONEER-HF 2019; PRIME 2019) with pooled odds ratios (ORs) of 0.83, 95% CI: 0.74–0.92 (p = 0.0006) and 0.78, 95% CI: 0.69–0.88 (p < 0.0001), respectively; they found no reduction of the odds of both outcomes when analysing patients with HFpEF, in particular 2 RCTs with ARB as the control group (PARAGON-HF 2019; PARAMOUNT 2012). These results are in the same direction as our analyses by type of control, as RCTs comparing S/V to ACEI were conducted in patients with HFrEF and RCTs comparing LCZ696 to ARB were conducted in patients with HFpEF. Regarding hospitalisation for HF in 5 RCTs (PARADIGM-HF 2014; PARAGON-HF 2019; PARAMOUNT 2012; PIONEER-HF 2019; PRIME 2019), Zhang et al. found that the pooled OR was 0.79, 95% CI: 0.72–0.85 (p < 0.00001) – also in the same direction as our comparison of S/V vs. ACEI or ARB together [30]. Compared with enalapril or valsartan together, S/V was associated with a higher risk of symptomatic hypotension (RR = 1.47, 95% CI: 1.34–1.60; 6 RCTs; p < 0.00001), lower risk of worsening renal function (RR = 0.81, 95% CI: 0.70–0.94; 6 RCTs, p = 0.005), and serious hyperkalaemia (≥ 6.0 mmol/l) (RR = 0.76, 95% CI: 0.65–0.89; 3 RCTs; p = 0.0007). We found similar risk of symptomatic hypotension but lower risk reduction in worsening renal function. Finally, we evaluated hyperkalaemia events of > 5.5 mmol/l, without differences between S/V and ACEI or ARB together, and their results are in concordance with ours.
According to the guidelines of the ACCF/AHA and the ESC, type B natriuretic peptides are the most valuable and reliable biomarkers for diagnosing HF and cardiac dysfunction as well as biomarkers of prognosis, severity, and treatment effectiveness assessment [1, 2, 31, 32]. NT-proBNP is not a substrate for neprilysin; therefore, it seems to be the best biomarker for monitoring the effects of S/V [33]. In addition to the beneficial clinical effects, S/V significantly decreased NT-proBNP levels in our study as well as in the meta-analysis of Wang et al. [27].
The full mechanism of action of S/V is unknown, but it exerts a positive modulation of the neuroendocrine balance, with enhancement of physiological diuresis and dilatation due to neprilysin inhibition by sacubitril [34]. In the biomarker sub-study of the PARADIGM-HF trial, the authors observed higher levels of urinary cGMP, the downstream effector of ANP and BNP, and lower plasma levels of NT-proBNP, reflecting a reduction in ventricular end-diastolic pressure [35]. cGMP, in turn, physiologically increases diuresis and vasodilatation and improves remodeling. In the observational, single-group study of Januzzi et al., reduction of NT-proBNP following treatment with S/V was associated with increases of LVEF of 9.4%, and reductions in indexed LV, LA volumes, and E/e′ [36]. In our meta-analysis, we found reductions of SBP and DBP within S/V compared to ACEI or ARB together, as well as to ACEI and ARB separately; however, the association between S/V and BP has not been found previously [37]. This association may be connected with potential benefits in patients with HF and high blood pressure values, especially with HFpEF.
Limitations of our study
We included 11 RCTs, outcome data were limited to 10 RCTs, and 3 weird RCTs (PARADIGM-HF 2014, PARAGON-HF 2019, PIONEER-HF 2019) contributed to most of the data of clinical outcomes [8, 19, 20]. The assessment of S/V effect on all-cause mortality and both HF and CV hospitalisations compared to ARB was based on the only PARAGON-HF study performed until now on the group of HFpEF patients [19]. Outcome data to examine heart systolic and diastolic heart function were also scarce and limited to subsamples of randomised individuals within RCTs of very low to moderate certainty of evidence. The composite outcome decline in renal function, which combined death from renal failure end-stage renal disease or > 50% decrease in eGFR from baseline, was reported in 2 large RCTs [8, 19]. All-cause hospitalisation only was reported in the Tumasyan RCT, which was only published as an abstract [23].
In conclusion, sacubitril/valsartan had positive effects on HF hospitalisation, CV mortality, and all-cause mortality in patients with HF in comparison to ACEIs, ARBs, or both. These effects were associated with reduction of NT-proBNP levels, reflecting a decrease in left ventricular diastolic wall stress, and in levels of hs-TNT levels, reflecting a decrease in myocardial injury. Also, S/V slightly reduced blood pressure and slightly improved quality of life. Our findings are applicable to the overall HF population independently of left ventricular ejection fraction or NYHA class, and for both chronic and acute HF. Given that we found beneficial effects on kidney function over time without increasing the risk of hyperkalaemia, S/V can be used on patients with HF and chronic kidney disease. Taking into account the higher risk of hypotension associated with S/V when compared to ACEI, ARB, or both, our results support the recommendation of S/V use until SBP is above 100 mm Hg. Further RCTs are needed to establish S/V effects on patients with HFmrEF and HFpEF and on other specific subpopulations such as diabetics, hypertensives, chronic kidney disease patients, and the elderly.
Conflict of interest
M.B. has received research grants/support from Amgen, Mylan/Viatris, Sanofi, and Valeant, and he has served as a consultant/received speakers fee from Amgen, Daiichi-Sankyo, Esperion, Freia Pharmaceuticals, Herbapol, Kogen, KRKA, Mylan/Viatris, Novartis, Novo-Nordisk, Polfarmex, Polpharma, Sanofi-Aventis, Servier, Teva, and Zentiva. He is CMO at the Nomi Biotech Corporation Ltd. A.M.B.-D. has given lectures that were sponsored by Novartis Polska Sp.z o. o. The remaining authors do not have any competing interests to disclose.