Introduction
Spinal cord injury (SCI) is caused by trauma or mechanical injury which affects the spinal cord’s integrity [1]. SCI results in multiple organ dysfunction and loss of motor and sensory function [2]. One of the major causes of permanent disability of patients is SCI in China. Of several pathways responsible for the development of SCI, one is primary injury caused by mechanical injury/trauma and secondary injury occurs due to involvement of different processes including inflammation, apoptosis, demethylation of neurons, and cytotoxicity [3]. There are several pathway involved in the degeneration of neurons including Notch and NLRP3 signaling [4]. Literature reveals that activation of Notch signaling occurs in spinal cord injury which leads to neurodegeneration by inhibiting the functional recovery of neurons [5]. Moreover, stimulation of the inflammasome activates the inflammatory cascade, which causes neuroinflammation [6]. NLRP3 activation promotes the innate immunity and inflammation, which activate the apoptosis of neuron cells [7]. cAMP response element-binding protein (CREB) family of transcription factors’ activation occurs by inducing a number of genes through CREB binding protein, reportedly regulating several biological functions including neuronal function [8]. The literature reveals that CREB contributes to the development of neurons and synaptic plasticity. Moreover, activation of CREB enhances the neurogenesis in cerebral ischemia induced neuronal injury [9].
There are several alternative medicines that have shown a beneficial effect against the management of chronic disorders. Tabersonine is isolated from Catharanthus roseus belonging to the family Apocynaceae [10]. It is chemically a terpene indole alkaloid reported to possess strong cytotoxic activity and also inhibits the formation of amyloid plaque [11]. The literature reveals that tabersonine prevents inflammation and injury of the lung by reducing the activity of macrophages and production of mediators of inflammation [12, 13]. Moreover, it regulates the NF-κB and p38/MK2 signaling pathways and protects against lung injury in LPS induced lung injured rats [12]. Thus the present study determines the protective effect of tabersonine against spinal cord injury.
Material and methods
Animals
Male SD rats of 200-230 g weight were kept under the standard guidelines (humidity: 60 ±5%; temperature: 24 ±3°C) for a 12-hour light and dark cycle. All the protocols of the study were approved by the institutional animal ethical committee of Nanchang University, China (IAEC/NU/2019/103).
Chemicals
Tabersonine was purchased from Sigma Aldrich Ltd., USA and ELISA assay kits were purchased from Thermo Fisher Scientific Ltd., USA. Antibodies used for the western blot and immunohistochemistry assay were purchased from Santa Cruz Biotechnology, USA.
Experimental
Allen’s method was performed for the establishment of the SCI model as per the previously reported method [14]. All the animals were anesthetized by injecting intraperitoneally 10% chloral hydrate and the spinal cord was exposed by making an incision on the midline skin. Rats were fixed on the platform and an impactor (2 mm diameter and 10 g weight) was dropped on the dorsal side of their spinal cord from 25 mm height. Later the incision was sutured and animals were transferred to cages for further observations.
Thirty-two animals were divided into four groups (n = 8): the control group having undergone sham SCI surgery; the spinal cord injured (SI) group having undergone SCI surgery and receiving saline solution for 10 days; Tabersonine 20 and 40 mg/kg group having undergone SCI surgery and receiving tabersonine 20 and 40 mg/kg, i.p. for the period of 10 days.
Estimations of neurological function
Analyses of neurological function were performed at 10 days after SCI. The Tarlov scale was used to estimate motor function, with the scores calculated as follows: 0, no movement of the lower extremity; 1, movement of the lower extremity without gravity; 2, movement of the lower extremity against gravity; 3, standing with assistance; 4, walking with assistance; and 5, normal.
Estimation of locomotor function
The Basso Beattie Bresnahan (BBB) locomotor rating scale was used to assess the locomotor function as per a previously reported study [15]. Determination of locomotor function was done at the end of the protocol. The score was calculated from 0 to 21, where a score of 21 was considered as normal locomotor function and a score of 0 was considered as complete paralysis.
Estimation of inflammatory cytokines
Homogenates of the spinal cord tissue samples were used to determine the levels of interleukin (IL)-1β, IL-6, and IL-16. Enzyme-linked immunosorbent assay (ELISA) kits were used to estimate the levels of inflammatory cytokines according to the manufacturer’s instructions.
Western blot assay
Assessment of expression of ERK1/2, CREB, IκB-α, NF-κB, caspase-3 and NLRP3 proteins was determined in the spinal cord tissue homogenate using western blot assay. A BCA assay kit was used to quantify the protein from the tissue homogenate and 10% SDS-PAGE was used to separate the proteins and they were transferred to a nitrocellulose membrane using an electroblotting technique. Subsequently, the membrane was blocked with a 5% blocking solution (non-fat milk) and then incubated in the blocking buffer with the following primary antibodies overnight at 4°C: ERK1/2, p-ERK1/2, CREB, p-CREB, IκB-α, p-IκB-α, NF-κB, caspase-3, NLRP3, NICD, Hes-1, Nestin and β-actin. Later goat secondary antibody conjugated with horseradish peroxidase (HRP) was added to the blocking buffer and a chemiluminescence kit was used to detect the proteins.
Real-time-polymerase chain reaction (RT-PCR) analysis
The RNA was isolated from spinal cord tissues using TRIzol reagent. The RevertAid First Strand cDNA Synthesis Kit (Fermentas, Ontario, Canada) was used to reverse-transcribe RNA. The primers mentioned below were mixed with RT2 SYBR Green Master Mix (Superarray, Frederick, MD, USA) to determine the gene expression using Quantitative SYBR Green PCR assays (Table I).
Table I
The primers of RT-PCR analysis
| Primer | Sense | Antisense |
|---|---|---|
| Notch-1 | 5’-CCAGTACAACCCGCTAAGGC-3’ | 5’-GGGACAAGGTATTGGTGGAGA-3’ |
| Hes-1 | 5’-GCGCCGGGCAAGAATAAATG-3’ | 5’-TCGGTGTTAACGCCCTCACAC-3’ |
Nissl staining
Spinal cord tissues were isolated from each animal and after dehydrating it tissues were seeded into liquid paraffin. A microtome was used to section the isolated spinal cord to 10 µm thickness and it was further stained with 1% cresyl violet stain. Image Pro Plus 6.0 software was used to estimate the Nissl stain positive neuronal cells.
Immunohistochemistry
Expression of NLRP3 in the spinal cord tissue was determined using immunohistochemistry assay as per the previously reported method [16]. Spinal cord tissue was embedded in paraffin by fixing it with 4% neutral buffered formaldehyde. Tissues were dehydrated using alcohol and 3% H2O2 was used to incubate with the tissue for 30 min at 37°C. Tissues were incubated with antibodies against NLRP3 at 4°C overnight and further tissues were incubated for 40 min at 37°C with biotin-conjugated secondary antibodies after washing the tissue with PBS. Tissues were stained for 10 min with DAB and counterstained with hematoxylin for 1 min. Sections were visualized using a Nikon Eclipse E800 microscope.
Results
Effect of tabersonine on neurological function
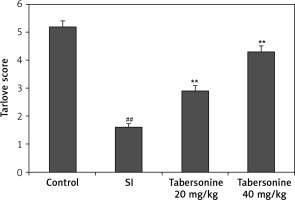
Effect of tabersonine on neurological function was assessed using the Tarlov scale in spinal cord injury rats as shown in Figure 1. The Tarlov scale score was significantly (p < 0.01) reduced in the SI group compared to the control group of rats. There was improvement in the Tarlov scale score in the tabersonine treated group compared to the SI group of rats.
Effect of tabersonine on locomotor function
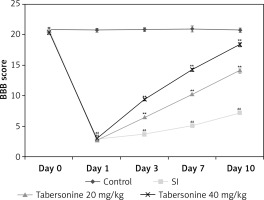
Figure 2 shows the effect of tabersonine on the locomotor function by determining BBB score in spinal cord injury rats on the 0th, 1st, 3rd, 7th and 10th day of the protocol. It was observed that the BBB score was significantly lower (p < 0.01) in all spinal cord injured groups than the control group of rats on the 1st day of protocol after induction of spinal cord injury. Moreover, on the 3rd, 7th and 10th day of the protocol, the BBB score was found to be (p < 0.01) lower in the SI group than the control group of rats. There was a significantly higher BBB score in the tabersonine treated groups than the SI group of rats.
Effect of tabersonine on inflammatory cytokines
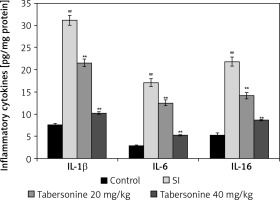
Mediators of inflammation such as IL-1β, IL-6 and IL-16 were estimated in the spinal cord tissue of tabersonine treated spinal cord injured rats as shown in Figure 3. Levels of IL-1β, IL-6 and IL-16 mediators were found to be enhanced up to 31.2, 17.1 and 21.9 pg/mg respectively in the SI group compared to the control group of rats. However, treatment with tabersonine significantly (p < 0.01) reduced the level of IL-1β, IL-6 and IL-16 mediators compared to the SI group of rats.
Effect of tabersonine on Notch signaling
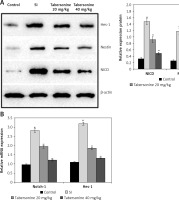
The effect of tabersonine on Notch signaling was estimated by determining NICD, Nestin and Hes-1 protein using western blot assay and mRNA expression of Notch-1 and Hes-1 using qRT-PCR in the spinal tissue homogenate of spinal cord injury rats. Expression of NICD, Nestin and Hes-1 protein was significantly (p < 0.01) higher in the spinal cord tissue of the SI group than the control group of rats. Moreover, mRNA expression of Notch-1 and Hes-1 was also enhanced significantly (p < 0.01) in the spinal tissue homogenate of the SI group compared to the control group of rats. It was observed that treatment with tabersonine attenuated the altered Notch signaling in the spinal tissue of spinal cord injured rats (Figure 4).
Figure 4
Effect of tabersonine on Notch signaling in spinal tissue homogenate of spinal cord injury rats. A – Relative expression of NICD, Nestin and Hes-1 protein by western blot assay, B – Relative mRNA expression of Notch-1 and Hes-1 by qRT-PCR
Mean ± SEM (n = 8), ##p < 0.01 compared to control group, **p < 0.01 compared to SI group.
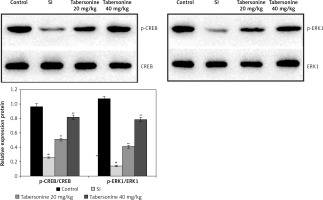
Effect of tabersonine on activation of CREB protein
The effect of tabersonine on activation of CREB protein was determined by estimating expression of CREB and ERK-1 protein in the spinal tissue homogenate of spinal cord injury rats as shown in Figure 5. There was a decrease in the expression of CREB and ERK-1 protein in the spinal tissue homogenate of the SI group compared to the control group of rats. However, expression of CREB and ERK-1 protein was significantly (p < 0.01) enhanced in the spinal tissue homogenate of the tabersonine treated group compared to the SI group of rats.
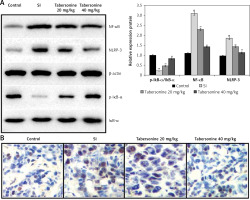
Effect of tabersonine on inflammasomes
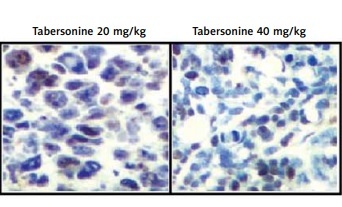
The effect of tabersonine on the inflammasomes was determined in the spinal tissue homogenate of spinal cord injury rats as shown in Figures 6 A and B. There was an increase in the expression of NF-κB and NLRP-3 proteins and a decrease in the expression of IκB-α protein in the spinal cord tissue of the SI group compared to the control group of rats. It was observed that treatment with tabersonine ameliorates the altered expression of IκB-α, NF-κB and NLRP-3 protein in the spinal cord tissue of spinal cord injured rats (Figure 6 A). Moreover, expression of NLRP3 was determined by IHC assay as shown in Figure 6 B. There was higher (p < 0.01) expression of NLRP3 in the spinal cord tissue of tabersonine treated group than the SI group of rats.
Figure 6
Effect of tabersonine on inflammasomes in spinal tissue homogenate of spinal cord injury rats. A – Relative expression of IkB-α, NF-kB and NLRP-3 protein by western blot assay, B – immunohistochemical analysis
Mean ± SEM (n = 8); ##p < 0.01 compared to control group, **p < 0.01 compared to SI group.
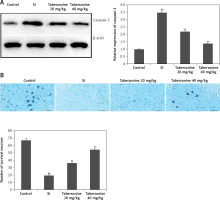
Effect of tabersonine on apoptosis of neuronal cells
The effect of tabersonine on the apoptosis of neuronal cells was determined by estimating the activity of caspase-3 enzyme and Nissl stain positive neuronal cells in the spinal tissue homogenate of spinal cord injury rats. Expression of caspase-3 protein was found to be enhanced significantly (p < 0.01) in the spinal tissue of the SI group compared to the control group of rats. There was lower expression of caspase-3 in the spinal tissue of the tabersonine treated group than the SI group of rats (Figure 7 A). The number of Nissl stain positive neuronal cells was lower (p < 0.01) in the SI group than the control group of rats. However treatment with tabersonine ameliorates the altered number of Nissl stain positive neuronal cells in the spinal tissue of spinal cord injured rats (Figure 7 B).
Figure 7
Effect of tabersonine on apoptosis of neuronal cells in spinal tissue homogenate of spinal cord injury rats A – Relative expression of caspase-3 protein by western blot assay, B – Frequency of survival of neurons
Mean ± SEM (n = 8); ##p < 0.01 compared to control group, **p < 0.01 compared to SI group.
Discussion
Spinal cord injury is one of the major causes of permanent disabilities throughout the globe including China. There are several pathogenesis pathways involved in the progression of secondary injury induced due to spinal cord injury. Management of spinal cord injury induced secondary injury is still a challenge for the medical field. The present study evaluates the protective effect of tabersonine against spinal cord injury. Tabersonine’s effect was determined by estimating locomotor and neurological function in spinal cord injured rats. Moreover, mediators of inflammation were estimated using ELISA and the effect of tabersonine on the Notch/inflammasomes signaling was estimated by RT-PCR, western blot assay and immunohistochemistry. Apoptosis of neuronal cells was estimated by staining with Nissl stain on spinal cord tissue in SCI rats.
Notch signaling contributes to the regulation of differentiation and proliferation of endogenous neural cells [17]. Notch is a transmembrane protein receptor which is activated by binding to DSL and also helps in the regulating of neurogenesis [18]. Notch intracellular domain contributes to the activation of Notch by regulating the expression of Nestin and Hes-1 protein [19]. Data of the study reveal that treatment with tabersonine ameliorates the altered Notch signaling in the spinal cord tissue in SCI rats. The literature reveals that the NLRP3 inflammasome plays an important role in the development of secondary injury in spinal cord injured rats [20]. Inflammasome inhibition was reported to reduce the production of mitochondrial ROS and also decrease the section of inflammatory mediators such as IL-1β, IL-6 and IL-16 [21, 22]. Thus inhibition of inflammasomes is a novel target for the development of drugs used for the management of SCI [23]. Results of the study suggest that the level of mediators of inflammation was significantly reduced in the tabersonine treated group compared to the SI group of rats. Moreover, treatment with tabersonine attenuates the altered expression of IκB-α, NF-κB and NLRP-3 protein in the spinal tissue of spinal cord injured rats.
There are several roles such as depression and stress that are controlled by the ERK-CREB signal pathway. The literature also reveals that activation of cAMP-response-element-binding protein (CREB) stimulated neurogenesis and could be used as a novel target for the development of drugs for the treatment of neurodegenerative disorders [24]. There are several factors such as mediators of inflammation and NMDA that have an effect on the inhibition of CREB [25]. There are several pathways involved in the development of neuronal injuries including Notch signaling. It is involved in the synaptic plasticity of neurons [26]. Notch signaling is involved in a number of neurodegenerative disorders [27]. Activation of Notch signaling downregulates the expression of CREB protein in neuronal injury and the drug used in the management of Alzheimer’s disease enhances the phosphorylation of CREB [28]. Data of the study suggest that treatment with tabersonine ameliorates the altered expression of ERK/CREB protein in the spinal cord tissue of spinal cord injured rats.
In conclusion, data of the investigation suggest that tabersonine protects against spinal cord injury by activating CREB and reducing NLRP3/Notch signaling. The results suggest that tabersonine could be used clinically for the management of spinal cord injury.