Tuberculosis is one of the oldest known infectious diseases and affects an estimated 2 billion people worldwide [1]. Spinal tuberculosis is a common manifestation of extrapulmonary tuberculosis and osteoarticular tuberculosis [2]. It can cause serious problems, such as kyphotic deformity, spinal cord compression and paraplegia, when it is not treated adequately [3]. With the development of anti-tuberculosis drugs, some cases of spinal tuberculosis can be cured by conservative treatment. However, for spinal cord compression, kyphosis and other serious conditions, surgical treatment is required [4].
Three-dimensional (3D) printing is a manufacturing process in which materials, such as plastic or metal, are deposited in layers to create a 3D object from a digital model. It has the advantage of enabling the fabrication of objects with complex freeform geometry [5]. This technology has been used in orthopaedics to improve the efficacy of surgery [5, 6]; however, there are few studies reporting the application of 3D printing in spinal tuberculosis treatment. In this paper, we report the case of a female patient with spinal tuberculosis for whom a 3D printing-assisted artificial vertebral body was used to reconstruct a diseased vertebra.
An 18-year-old Chinese female patient presented with neck and flank pain for over 10 months. Other symptoms included low-grade fever and repeated night sweats. Imaging examination in another hospital showed the destruction of multiple vertebral bodies, as well as paravertebral abscesses. Thus, a diagnosis of spinal tuberculosis was made. Conservative treatment with anti-tuberculosis drugs (isoniazid and rifampicin) was performed, but the symptoms persisted. For further treatment, the patient was admitted to our hospital.
Physical examination showed no obvious deformity of the spine. Laboratory tests revealed that leucocytes and C-reactive protein were elevated (to 11.02 × 109/l and 123.11 mg/l, respectively) and that the erythrocyte sedimentation rate was significantly accelerated (to 60 mm/h). Furthermore, T-SPOT was positive. Imaging examination revealed the destruction of T1–T2, T6–T8, T12–L1, L3 and L5–S1 and the formation of multiple intensive abscesses at T1–T2, T6–T8, T12–L1 and L3 and in the thoracic cavity (Figure 1). Due to these findings, the diagnosis of spinal tuberculosis was confirmed. The anti-tuberculosis drugs isoniazid, rifampicin and amikacin were administered. After 2 months of standard anti-tuberculosis treatment, the symptoms of the patient were significantly alleviated. Thus, surgical treatment was performed.
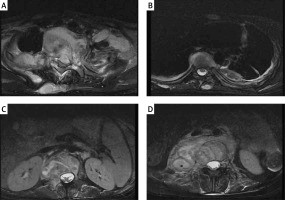
Figure 1
The MRI of an 18-year-old woman preoperatively. A – The destruction of T1-2, B – the destruction of T7, C – the destruction of L1, D – the destruction of L3
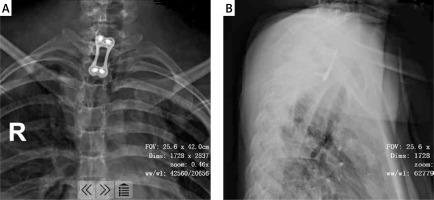
As the patient had destruction in multiple vertebral bodies, as well as several abscesses, a three-stage surgical procedure was adopted. The aim of the first stage was to clear the tubercular infection focus at T1–T2. Using an anterior approach, we cleared the abscesses located in the cervical region. Then, we removed the C7–T1 and T1–T2 intervertebral discs and resected the remaining vertebrae of T1. After that, we performed an ilium plate placement. A titanium plate was closely attached to C7 and T2 and four screws were inserted to attach it to the ilium (Figure 2). The second stage of the surgery involved internal fixation of the thoracic, lumbar and sacral vertebrae. We made a posterior midline incision from T6 to T8. Six pedicle screws were inserted into each side of T6–T8, and the necrotic tissue in the paravertebral muscles was simultaneously cleared. Then, we made a posterior midline incision from T11 to S1. Pedicle screws were inserted into T11–S1. Once the L5–S1 vertebral plates were exposed, we removed the L5–S1 intervertebral disc and cleared the anterior sacral abscess. After that, we performed an autograft to promote fusion. The final procedure in the second stage was to clear the abscesses located at T3–T8 and in the thoracic cavity through thoracotomy.
Figure 2
The fixation of C7-T2. The titanium plate was closely attached to C7 and T2, and 4 screws were inserted to fix the ilium plate
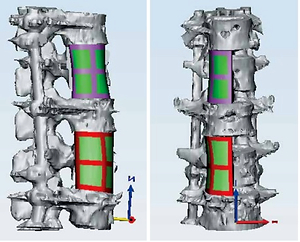
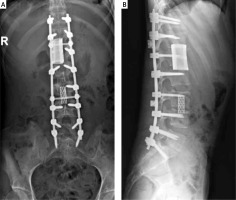
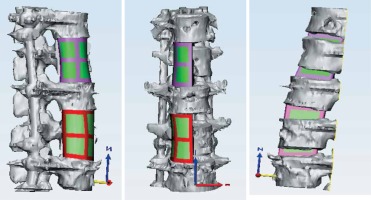
The third stage of the surgery aimed to clear the tubercular infection foci of T12–L1 and L3. Because the destruction of the vertebral bodies was extensive, a 3D printing-assisted artificial vertebral body replacement was considered appropriate. Preoperatively, we used Mimics 19.0 software (Materialise, Leuven, Belgium) to construct a 3D model of the patient’s diseased vertebra from computed tomography images. The diameter and height of the integrated artificial vertebral body were calculated by computer simulation. We designed artificial vertebral bodies of two different heights to provide options. The computer simulation results showed that the edges of the artificial vertebral body were flush with the upper and lower vertebral edges and that the surface fit was suitable (Figure 3). We printed the final product and the surgery was conducted. The anteroposterior diameter of the artificial vertebral body was 2.8 cm, the axis length was 2.1 cm and the height options were 4.2 cm and 4.4 cm (Figure 4). We chose the right external peritoneal approach, and about 10 cm of the 11th right rib was resected. We carefully cleaned the tuberculosis focus, removed the T12–L1 and L1–L2 intervertebral discs and resected the diseased vertebrae L1 and L3. Then, the integrated artificial vertebral body was implanted into L1. During the insertion process, the total blood loss was about 1,600 ml. Thus, we abandoned the artificial vertebral body for L3 reconstruction. Instead, titanium mesh was used. A postoperative C-shaped X-ray suggested that the replacement was satisfactory (Figure 5).
Figure 3
The simulations prior to the surgery. The edges of the artificial vertebral body were flush with the upper and lower vertebral edges and the surface fit was suitable
After three-stage surgery, the patient continued to receive anti-tuberculosis drugs and her preoperative symptoms were significantly alleviated. Two months later, the patient could walk with the assistance of a brace.
In this study, we report the case of a female patient with spinal tuberculosis involving the destruction of multiple vertebral bodies, as well as paravertebral abscesses. She underwent a three-stage surgical procedure, and a 3D printing-assisted artificial vertebral body replacement was used to reconstruct her L1. The results indicate that 3D printing technology can be used in surgery for spinal tuberculosis.
Spinal tuberculosis is a common manifestation of extrapulmonary tuberculosis that can cause paralysis and increase mortality in patients [2]. The purpose of surgical treatment for spinal tuberculosis is to clear tuberculosis foci, remove spinal cord compression, reconstruct the biomechanics of the vertebra and increase spine stability [7, 8]. In our case, the patient had destruction of multiple vertebral bodies, as well as paravertebral abscesses, and presented with neck and flank pain. Surgery was necessary for the patient. Due to the wide range of vertebral bodies displaying destruction, we adopted a three-stage surgical procedure using a combination of anterior and posterior surgery. It has been reported that an anterior approach can achieve complete debridement, decompression and bone graft fusion and prevent posterior column destruction [9]; however, it is not effective for the correction of kyphosis [8]. Thus, a combination of anterior and posterior surgery is recommended in some studies [8]. In our case, we first used an anterior approach to clear the tuberculosis focus at T1–T2. Autologous iliac bones were placed in the defective area of the vertebral body. Second, we used posterior surgery to fix the thoracic, lumbar and sacral vertebrae and clear the tuberculosis focus at L5–S1. In the final stage, the tuberculosis foci at T12–L1 and L3 were cleared.
In this last stage, we used a new strategy involving a 3D printed model to improve the stability of the spine. The 3D printed model was constructed using digital orthopaedic technology to simulate the optimal surgical design. To date, this technology has been used in spine surgery, joint replacement and bone tumour resection [10, 11]. The application of 3D printing technology in the treatment of spinal tuberculosis has also been reported in some literature. Wang et al. retrospectively analysed data from 60 patients with thoracic tuberculosis and found that 3D printing technology combined with guide plates effectively reduced bleeding and increased the safety and accuracy of nail placement [12]. Zhang et al. reported a case of cervical tuberculosis [7] in which, after 3D printing-assisted anterior debridement and artificial vertebral body replacement, the patient’s tuberculosis focus was completely cured [7]. Wang reported that 3D printing technology was useful in patient-specific preoperative planning for a patient with thoracic spinal tuberculosis [13]. In our case, we used a 3D printing-assisted artificial vertebral body to replace the patient’s diseased L1 vertebrae. A postoperative C-shaped X-ray suggested that the replacement was satisfactory. Preoperatively, we had planned to use 3D printing-assisted artificial vertebral bodies to replace both L1 and L3. However, during the replacement of L1, the total blood loss was about 1,600 ml, exceeding the estimate of 800 ml. Thus, we abandoned the artificial vertebral body for L3 reconstruction and instead used titanium mesh. The preoperative computer simulation results showed that the edges of the artificial vertebral body were flush with the upper and lower vertebral edges and that the surface fit was suitable; however, the surgical procedure could not meet the ideal conditions for osteotomy. During the process, additional normal bone mass was removed in order to insert the artificial vertebral body. After surgery, we improved the artificial vertebral body we had made, and the anteroposterior diameter of the artificial vertebral body was shortened.
In conclusion, in this paper, we have reported the case of a female patient with spinal tuberculosis involving the destruction of multiple vertebral bodies, as well as paravertebral abscesses. Due to the wide range of vertebral bodies displaying destruction, we adopted a three-stage surgical procedure using a combination of anterior and posterior surgery. Additionally, 3D printing-assisted artificial vertebral body replacement was used to reconstruct L1. The results indicate that 3D printing technology can be used in surgery for spinal tuberculosis.