Introduction
Artificial urinary sphincter (AUS) implantation is the treatment of choice for male urinary incontinence (UI) [1]. The basic element of AUS that provides continence is a cuff that occludes the urethral lumen. It is usually placed around the corpus spongiosum at the bulbous urethra due to easy access and the possibility to be used in nearly all patients [2]. However, this technique is not free from disadvantages, mainly due to the fragility of the corpus spongiosum and the phenomenon of urethral atrophy. The second surgical technique applied in patients with a preserved prostate is implantation of the cuff around the bladder neck [3]. Since the first operations of this type were ineffective, we decided to place the cuff around the prostate as an alternative method. Very good initial functional outcomes (continence quality) encouraged us to continue using this method of AUS implantation.
Material and methods
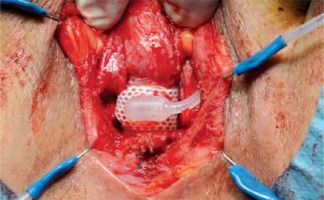
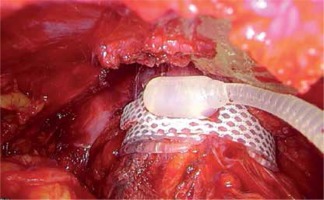
The clinical material was based on a group of 43 men, aged between 25 and 84 (mean age = 64.62 years), who had an AUS implanted due to severe UI in 1997–2015. The implanted device was an AMS 800 (American Medical Systems, now part of Boston Scientific Corp., Marlborough, MA, USA). Urinary incontinence was a consequence of transurethral resection of the prostate (TURP) in 23 (53.49%) patients and 8 (18.61%) patients after open prostatectomy. The remaining causes (Table I) constituted 12 (27.9%) cases. Most of our patients presented total UI and only some of them had severe stress UI (< 600 ml/day). The diagnostic pathway and selection for UI treatment included cystoscopy in order to assess the anatomy and function of the lower urinary tract. Additionally, in Group 1 a urodynamic study was carried out to assess detrusor contractility and transrectal prostate ultrasonography for prostate volume measurement. After contraindications to AUS implantation have been ruled out [4], written consent to the procedure was obtained. The perineal approach was used to place the cuff around the bulbar urethra [5]. The procedure during which the cuff was implanted around the prostate was as follows: a lower midline incision was made to access the retropubic space. The prostate was exposed from the fat, and subsequently the endopelvic fascia was incised. In the middle part of the prostate on both sides within neurovascular bundles, a 1.5 cm wide tunnel was created on the posterior surface of the prostate by blunt and sharp dissection. The integrity of the rectum was inspected by gas introduced through a catheter and observation of the operating field filled with saline. The cuff of an appropriate length was placed around the prostate. Subsequently, the remaining elements of the sphincter were implanted in the typical way. The manner of sphincter preparation and connection was not different from the technique involving the perineal approach. After 6 weeks, the sphincter was activated and the patients were instructed how to use it. Continence and the ability to use the sphincter were assessed directly after AUS activation. Outpatient follow-up visits occurred every 3 months in the first year and subsequently once a year. Continence after the procedure was assessed on the basis of a 24 h pad test and an opinion expressed by the patients according to the following criteria: complete continence (negative pad test), good level of continence (volume of urine leakage 10 ml daily), poor level of continence (> 10 ml to < 100 ml daily) and incontinence (≥ 100 ml daily). The AUS cuff was implanted around the bulbar urethra in 23 (53.48%) patients, around the prostate in 20 (46.51%) cases (Figures 1–4).
Table I
Causes of urinary incontinence in patients operated on using artificial urinary sphincter and the location of the cuff
Figure 1
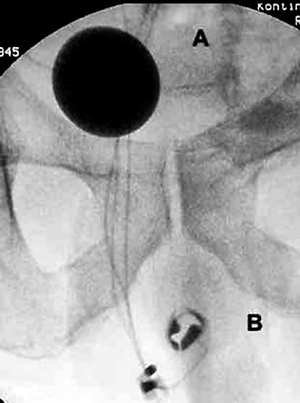
Radiographic images of AMS 800 implanted on bulbar urethra: A – a balloon in the pelvis, B – cuff around the bulbous urethra, C – control pump in the scrotum
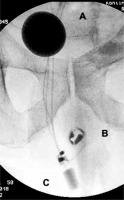
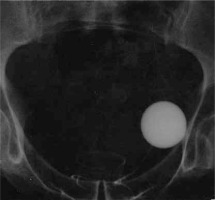
Figure 3
Radiographic images of the AMS 800 implanted around the prostate: A – a balloon in the Retzius space, B – the cuff size 10 cm around the prostate
Statistical analysis
To compare mean values and the two analysed methods of implantation, a nonparametric approach was used, as embedded within the PROC NPAR1WAY procedure. We used the logistic regression analysis as supplied in the PROCLOGISTIC procedure to assess the factors related to post-procedure urinary continence.
Results
Detailed information on the causes of urinary incontinence and the surgical technique applied (the site of cuff implantation) is presented in Table I. Demographic data of patients from these groups are presented in Table II.
Table II
Characteristics of the comparison groups.
| Group | Cuff location | Number of patients | Age/SD | Median follow-up (SD) | Size of the cuff |
|---|---|---|---|---|---|
| 1 | Prostatic urethra | 20 | 66.1/11.1 | 101.2 (4.9–184.3) | 9.2 |
| 2 | Bulbar urethra | 24 | 59.3/16.3 | 88.8 (7.9–146.6) | 4.9 |
There were no cases of perioperative mortality or death in the early postoperative period. The median follow-up for all patients was 67 months (1.1–191.4). The median values for individual groups were: 101.2 months (4.9–184.3) for Group 1, 88.8 months (7.9–146.0) for Group 2. The difference between Groups 1 and 2 is not as prominent. Complications did not develop in 26 (59.1%) patients. One complication was noted in 16 (36.4%) patients, 2 complications in 2 (4.5%) patients. The median time to complications in Group 1 was 90.3 months (30.6–154.9) and in Group 2A it was 10.7 months (0–132.7) (p = 0.007). Complications were divided based on their etiology into 3 types – mechanical, medical and iatrogenic – and are listed in Table III. Grade of complication in the Clavien-Dindo classification is added in brackets.
Table III
Complications after artificial urinary sphincter implantation*
Complications requiring reintervention occurred in 14 (31.8%) patients: in 20% of patients from Group 1 and in 41.6% of patients from Group 2 (p = 0.001). The most common complications in Group 2 were erosions with or without infection (4 patients) as well as urethral atrophy (4 patients). The latter was not observed in Group 1. To sum up all complications associated with the bulbar urethra, they occurred in 9 cases (37.3%) in Group 2, and in 2 (10.0%) cases in Group 1 (one intraoperative injury with no further consequences and one iatrogenic injury due to the lack of system deactivation after 18F catheter placement). Some patients from both groups developed transient urinary retention after catheter removal, which subsided spontaneously after several days up to 2 weeks. Another significant element of the analysis was the quality of continence assessed on the basis of the interview and pad test during consecutive follow-up visits. The classification criteria to individual continence categories have been mentioned in the Material and methods section. Complete continence was obtained in 16 (80%), and 8 (33.3%) patients from Group 1, 2, respectively. The difference between Groups 1 and 2 was statistically significant, p = 0.002. When considering fully continent patients and patients with a good level of continence together, the number of such patients was 20 (100%) in Group 1 compared with 70.8% in Group 2. For the comparison between Groups 1 and 2, p = 0.008. All data are included in Table IV.
Table IV
Characteristics of postoperative continence in groups
[i] Group 1 – patients with the cuff placed around the prostatic urethra. Group 2 – patients with the cuff placed around the bulbar urethra.Complete continence (negative pad test), good level of continence (volume of urine leakage 10 ml daily), poor level of continence (> 10 to < 100 ml daily) and incontinence (≥ 100 ml daily).
Discussion
This paper is the first report comparing continence and complications after AUS implantation using two surgical techniques: perineal vs. periprostatic approach. One of the few papers on the outcomes of a similar technique of AMS 800 implantation around the bladder neck in men was published by Venn et al. in 2000 [3]. In 2013, another paper on the outcomes of implantation around the bladder neck in men was published; it concerned 51 patients from 4 academic urology wards followed for 83 months [6]. For reliability reasons, the analysis was conducted in two groups comparable in terms of etiology, age, incontinence severity and duration of follow-up: Group 1 (prostatic urethra) and Group 2A (bulbar urethra). All patients presented with total or severe UI (< 600 ml/day) and the pre-operative status did not influence functional outcomes. Complete continence in the patients with periprostatic cuffs (Group 1) was noted in 16 (80%) men, who needed no pads. As for the patients with a cuff placed around the bulbous urethra (Group 2), such an outcome was observed in 8 (33.3%) cases, p = 0.002. Complete continence without the need to use any pads has a considerable impact on the quality of life after AUS implantation [7]. Such results are obtained in 4.3–85% of patients in various reports [8, 9]. However, in most cases, the rate is not higher than 50% [10]. Furthermore, during the follow-up, there was no significant continence deterioration without complications, which was also observed by Kim et al. during a 10-year postoperative observation [11]. The results obtained attest to considerably better continence quality after periprostatic cuff implantation. This difference may have a number of causes. The first is the manner of cuff selection, which is conducted with a much greater force. Tight cuffs can more frequently lead to temporary urinary retention directly after surgery, but they also make it possible for the patient to be continent without system activation due to the formation of some degree of bladder outlet obstruction [12]. That is why the risk of urinary retention increases in patients with a weakened detrusor muscle. It is therefore important to conduct a urodynamic study in order to assess the strength of detrusor contraction before a decision about AUS implantation. Thanks to strong connective tissue stroma and considerable thickness of the prostatic urethra, the risk of atrophy with subsequent erosion (because of a tight AUS cuff) is minimal, which was also shown in the follow-up. Only 1 case was noted, 8 years after surgery. It was caused by keeping a catheter for too long without AUS deactivation (iatrogenic complication). By comparison, 9 such cases were noted in Group 2A and they occurred after approximately 8.9 months. Median time to the development of the first complication was 90.3 months (range from 30.6 to 145.9 months) in Group 1 and 10.7 months (0–132.7 months) in Group 2A; p = 0.001. This difference may be a consequence of complications associated with AUS infection occurring within the first 24 months after surgery in Group 2A, which are lacking in Group 1 [13, 14]. That is why patients with an AUS implanted periprostatically could use the sphincter for a longer period of time, which undoubtedly translates into patient satisfaction. The rate of complications was 35% in Group 1 and 54.2% in Group 2A, p = 0.001. Some of them, e.g. transient urinary retention or postoperative haematoma, required no surgical intervention. Complications that require revision surgery, which usually entails replacement of a single element of the sphincter or the entire system, are particularly significant. Such situations were observed in 30% of the patients from Group 1 and in 45.8% of the patients from Group 2A, p = 0.001. The rate of complications reported by other authors ranges from 18 to 44.8% [10, 11]. In the light of these data, our results fall within the ranges reported by others. Of note is the fact that markedly better outcomes in terms of the time to complications were observed in Group 1. The most common complications were associated with the bulbar urethra embraced by the cuff: either erosion with or without infection, or atrophy leading to poorer continence. These problems concerned a total of 9 (37.5%) patients from Group 2A. Erosion with infection is usually caused by an iatrogenic error during urethral dissection or failure to maintain surgical field sterility [12]. Sterile erosions and atrophy, which can occur later than infectious complications, might be caused by poor cuff selection, poor urethra vascularisation, e.g. in patients after radiotherapy for prostate cancer, and in patients with fragile, delicate anatomical structures [15, 16]. Atrophy is frequently the most common isolated cause of complications [13]. A typical mechanical damage of 4–5 cm cuffs is their rupture at the site of bulb folding, which occurs on average after 68.9 months [17]. Due to a different anatomic structure and diameter of the prostate, cuffs of 8–11 cm are usually used. In our material, the average cuff length was 9 cm. This way, the activity of the cuff around a large circumference does not entail the risk of excessive folding of the delicate pad. This is reflected in the number of mechanical complications of this type in Group 2A (4 patients) and the lack of such problems in Group 1. A markedly lower number of complications as well as a longer time to their occurrence also contributes to better economic outcomes. Another typical complication in patients with a cuff placed around the prostatic urethra is urinary retention occurring a long time after implantation (after 5 years in the investigated patients) due to prostatic adenoma growth. In such cases, TURP was successfully performed. To date, two procedures of this type have been conducted effectively in patients with AUS. We observed no complications after the procedures, especially continence deterioration, erosion of the cuff and infections of the AMS system. The disadvantages of retropubic surgery also include: longer operative time, technical difficulty, risk of rectal injury and erectile dysfunction. However, there were no cases of erectile dysfunction directly after surgery in our material. Some patients from the study of Trost and Elliot [17] had weak erectile function before AUS implantation. That is why current data do not enable unequivocal assessment of a possibly negative influence on potency [17]. Another problem is the need to assess the size and circumference of the prostate since the longest cuffs are 11 cm long and may not be sufficient to embrace a large adenoma. Transrectal ultrasonography is essential to estimate the prostate size and, if needed, TURP needs to be performed prior to AUS.
Another potential complication during artificial sphincter implantation is seminal vesicle injury during dissection of the tunnel on the posterior surface of the prostate. We did not observe symptoms of seminal vesicle injury during surgery as, due to the nature of the procedure, we did not have direct visual control. On the other hand, seminal vesicles are located nearer to the midline and are closely connected with the prostate. Hence blunt gentle dissection with a finger seems safe. Another problem is associated with a more difficult diagnosis of mechanical failure of the cuff or balloon. In our practice, explantation and implantation of a new device during one procedure was conducted without a problem. But if the system has been removed because of erosion or infection and new implantation can be performed after 3 months, it will be an extremely difficult procedure. We have not performed such surgery yet. Diagnosis and treatment of complications is yet another problem after periprostatic implantation where both the cuff and the balloon are located in the retroperitoneal space. In the case of mechanical damage of one of these elements, replacement in our practice proceeded with no problems. However, in the case of, for example, infection when the entire system needs to be removed, and reimplantation can be performed only after 3 months, the procedure is then very difficult and dangerous. In order to minimise surgical trauma, we are currently trying to conduct laparoscopic procedures using our own surgical technique as described by Chłosta et al. [18].
The weakness of our study is its retrospective nature and small groups of patients. For that reason, it is not possible to formulate ultimate recommendations. However, the results described above indicate that implantation of the AUS around the prostate may be justified in selected cases since the quality of continence is markedly better and complications develop more rarely and later. In addition, sports, including cycling, are not contraindicated after such a procedure, which could be relevant for young physically active patients. Moreover, no accidental urine leakage was observed when sitting down. Periprostatic cuff replacement, like its implantation, is more difficult than its placement around the bulbar urethra. However, the cuff can be implanted into the same location without a risk of atrophy or deteriorated continence. Nevertheless, no guidelines on how to measure the cuff in such cases have been developed so far to ensure optimal adjustment. Further, prospective studies on larger series of patients are needed to confirm our encouraging results.
In conclusion, the analysis indicates that cuff placement around the prostatic urethra results in better continence and is characterised by fewer complications. This method is dedicated for patients who have not had the prostate gland removed. Due to the retrospective nature of this analysis and small groups of patients, it is not possible to formulate ultimate recommendations.