Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease of unknown etiology which causes progressive deterioration of the joints, leading to severe pain and functional disability [1]. The global prevalence of RA is calculated to be 0.5–1.0% of the population, and is two to four times higher in women than in men across all age groups [2]. The etiology of RA is not yet understood, since diverse risk factors of both environmental and genetic origin are involved in its onset and development [3–5]. Smoking is one of the principal factors of environmental risk. Smokers are twice as likely to develop RA, because tobacco increases production of the citrullinated proteins involved in the action mechanism of the disease [6, 7]. Pollution, stress, diet, and drinking alcohol are also environmental risk factors in RA [8, 9]. Similarly, higher socioeconomic and education levels are associated with a lower risk of RA [10].
Different studies have demonstrated the association between vitamin D and risk of RA. Low vitamin D levels have been associated with higher prevalence of RA [11]. Vitamin D is associated with skeletal metabolism, since it plays a fundamental role in bone mineral homeostasis through its involvement in regulating calcium and phosphate absorption [12]. Vitamin D therefore acts by boosting innate immune processes and reducing the effect of the adaptive immune system, which may lessen the risk of autoimmune diseases such as RA.
One way of studying the role of vitamin D in the immune system as a whole, and in RA in particular, would be to study the proteins and enzymes involved in the action mechanism of RA, such as vitamin D receptor (VDR) and its different polymorphisms [13, 14]. Vitamin D receptor is a nuclear transcription factor which regulates multiple genes responsible for inflammatory processes and immune system response [15]. The signaling pathway of VDR therefore plays a crucial role in the manifestation of autoimmune diseases. It has been found that different immune system cells express the VDR gene [16]. This expression is found in macrophages, monocytes, and activated T lymphocytes (CD4+, CD8), chondrocytes and synovial cells in the synovial membrane and cartilage in RA patients [16]. In this disease, T lymphocytes, macrophages and activated plasma cells infiltrate joints [17]. Th1 lymphocytes associated with inflammatory processes are involved in the development of the disease, as are pro-inflammatory cytokines, which leads to the production of TNF-α and the destruction of bone and cartilage [18]. All of this suggests that the VDR gene and its polymorphisms have an effect on disease immune regulation and joint inflammation.
The VDR gene is found on chromosome 12q13.11 and is highly polymorphic [7, 19]. Numerous polymorphisms have been described for this gene, the most widely studied of which are: FokI (rs2228570, exon 2, +30920 C > T, Met→Thr), BsmI (rs1544410, intron 8, + 63980 G > A), TaqI (rs731236, exon 9, +65058 T > C, Ile→Ile), ApaI (rs7975232, intron 8, +64978 C > A) and Cdx2 (rs11568820, exon 1, +1270 G > A). Studies have demonstrated that the different polymorphisms found in the VDR receptor may cause receptor dysfunction and variations in the immune response, and similarly in all of the processes regulated by this receptor. Uitterlinden et al. found that VDR polymorphisms BsmI and TaqI created a silent codon which therefore had no effect on the protein structure, but could be associated with greater VDR gene mRNA stability [20, 21]. As regards the FokI polymorphism, this is located in the start codon, giving rise to a new initiation site and affecting the structure of the molecule, since there is a change in the size of the synthesized protein which is in the shorter form and has higher transcriptional activity [22]. All of these variations can affect the VDR as regards its structure and function, and therefore also affect vitamin D function as demonstrated in recent studies which have shown that VDR gene polymorphisms are associated with bone loss in RA [21, 23, 24]. Different studies have been undertaken to seek the relationship between the VDR gene and RA, amongst which a meta-analysis was carried out on Caucasian and Asiatic study populations which linked the VDR gene FokI (rs2228570) polymorphism to the presence of RA in the study population and concluded that low levels of vitamin D may be a risk factor for RA [25]. Another study by Goertz et al. in Germany found a relationship between the Bsml genotype and the occurrence of RA [14, 26].
In view of the foregoing, we carried out this study to assess the effects of VDR gene polymorphisms (FokI (rs2228570), BsmI (rs1544410), TaqI (rs731236), ApaI (rs7975232) and Cdx2 (rs11568820) on the risk of developing RA.
Material and methods
Study subjects
A retrospective case-control study was performed. This study included 214 RA patients and 748 control subjects of Caucasian origin, the case/control ratio being 3. Cases were diagnosed as RA at Virgen de la Nieves University Hospital in Granada, Spain, between 1980 and 2013. Control subjects were Caucasian persons over 18 years old. The subjects participating in this study gave their informed consent in writing for the extraction of blood and saliva samples. This study was conducted in accordance with the Helsinki Declaration and with the approval of the Andalusian Public Health System Biobank Ethics Committee.
Sociodemographic and clinical variables
Sociodemographic data included gender, tobacco use, age at diagnosis and duration of illness. The clinical condition of patients when diagnosed with RA was described using the DAS28 clinical parameter and the RF biochemical parameter. These data were obtained from the hospital databases. Tobacco use was classified into current smokers, former smokers and never smokers. Individuals were classified as never smokers if they had never smoked or smoked < 100 cigarettes in their life; as former smokers if they had smoked ≥ 100 cigarettes in their life but did not currently smoke, and as current smokers if they had smoked ≥ 100 cigarettes in their life and still smoked.
Genetic variables
DNA isolation
DNA samples isolated from samples of blood or saliva were obtained from the Virgen de la Nieves University Hospital Biobank, which is part of the Andalusian Public Health System Biobank. Blood samples (3 ml) were collected in BD Vacutainer K3E Plus Blood Collection Tubes. Saliva samples were collected in 50 ml conical BD Falcon tubes (BD, Plymouth, United Kingdom). DNA isolation was performed using the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions for DNA purification from blood or saliva and stored at –40°C.
Detection of gene polymorphisms
VDR gene FokI, BsmI, TaqI, ApaI and Cdx2 polymorphisms were analyzed by real-time PCR using TaqMan probes. The assay ID used for gene FokI (rs2228570) is C_12060045_20, for BsmI (rs1544410) is AN324M4, for TaqI (rs731236) is C_2404008_10, for ApaI (rs7975232) is C_28977635_10 and for Cdx2 (rs11568820) is C_2880808_10. Genotyping analysis was performed using real-time polymerase chain reaction (PCR) allelic discrimination TaqMan genotyping assays (ABI Applied Biosystems, 7300 Real-Time PCR System) based on manufacturer protocols.
Statistical analysis
Descriptive analysis was performed using R 3.0.1 [27]. Quantitative data were expressed as the mean (± standard deviation) for normal-distribution variables or medians and percentiles (25 and 75) for non-normal-distribution variables. The Shapiro-Wilk test was used to assess normality.
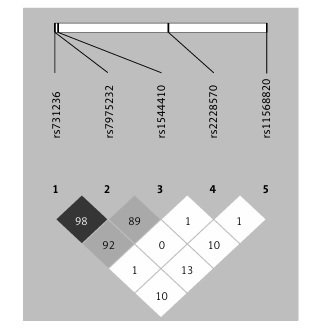
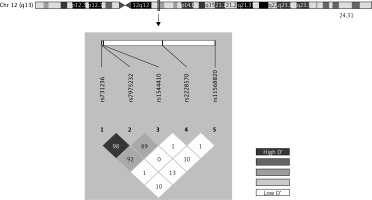
The Hardy-Weinberg equilibrium and frequency of paired haplotypes were determined, and Lewontin’s D prime (D’) and linkage disequilibrium coefficient (r2) were calculated. The bivariate correlation between risk of RA and polymorphisms was evaluated for multiple models (genotypic, additive, allelic, dominant and recessive) using Pearson’s χ2 test and Fisher’s exact test to give the odds ratio (OR) and 95% confidence interval (CI95%). The models were defined as follows: allelic (D vs. d), dominant ((DD, Dd) vs. dd), recessive (DD vs. (Dd, dd)) and genotypic (DD vs. Dd, Dd vs. dd) and additive, D being the minor allele and d the major allele. Adaptative permutation analysis (EMP1), which compares the observed statistic with 100,000 permutation statistics obtained for the polymorphism in question, was used for multiple comparisons. Unconditional multiple logistic regression models (genotypic, dominant and recessive) were used to determine the influence of possible confounding variables on the risk of RA. All of the tests were bilateral with a significance level of p < 0.05, and were performed using the free open source R 3.2.2 or PLINK toolset for whole genome association analysis [27–29]. Linkage disequilibrium (LD) was also developed with Haploview 4.2 software and haplotype analysis was performed based on the previous literature with SNPStats, a web tool for the analysis of association studies [30–34].
Results
Patient characteristics
The mean age of patients was 45 years [35, 53]. There were 170 women (170/214, 79.44%) and 44 men (44/214, 20.56%). The median disease duration was 17 years [11, 22.75], the RF value was above 48 in 108 patients (108/214, 50.47%) and the DAS28 value was below 5.4 in 110 patients (110/214, 51.4%). The control group was made up of 342 (45.12%) women and 416 (54.88%) men. The median age of control subjects was 64 years [44, 75]. The clinical, sociodemographic and pathologic characteristics of the 214 RA patients and the 748 control subjects are given in Table I. Cases and controls presented significant differences according to gender (p < 0.01; OR = 0.21; 95% CI = 0.14–0.30; men vs. women; Table I), age (p < 0.01; OR = 2.84; 95% CI = 2.08–3.89; < 45 years old vs. > 45 years old; Table I) and tobacco use (p < 0.01; OR = 5.16; 95% CI = 2.78–10.24; current smokers vs. former smokers and p < 0.01; OR = 5.98; 95% CI = 3.43–11.31; non-smokers vs. former smokers; Table I).
Table I
Clinico-pathologic characteristics of rheumatoid arthritis cases and controls
Genotype distribution
Genotype frequencies matched expected values as per the Hardy-Weinberg equilibrium (HWE) model, with the exception of rs1544410 and rs2228570 (Supplementary Table SI). The D’ linkage disequilibrium and r2 values are given in Supplementary Table SII and Figure 1 shows a graph for LD. All of the polymorphisms presented minor allelic frequencies of above 1% and therefore none of them was excluded from the analysis (Supplementary Table SIII). Haplotype frequency estimations are contained in Supplementary Table SIV.
Influence of gene polymorphisms on risk of rheumatoid arthritis
Bivariate analysis was carried out on multiple models: genotypic, additive, allelic, dominant and recessive (Supplementary Table SV). VDR FokI (rs2228570) was the only polymorphism which showed a trend for risk of RA in genotypic and recessive models (Supplementary Table SV). This trend was also found after correction by permutation analysis (Supplementary Table SVI). The genotypic model showed that patients carrying the AA genotype were at lower risk of RA (p = 0.0613; OR = 0.76; 95% CI = 0.46–1.23; AA vs. GG; Supplementary Table SVI), whilst carriers of the AG genotype were at risk of RA (p = 0.0613; OR = 1.29; 95% CI = 0.94–1.80; AG vs. GG; Supplementary Table SVI). Similarly, the recessive model showed that patients with the AA genotype were at lower risk of RA compared with patients with the G allele (p = 0.0980; OR = 0.66; 95% CI = 0.41–1.03; Supplementary Table SVI).
This trend was confirmed in the logistic regression analysis (Table II). The recessive logistic regression model adjusted by gender, age and tobacco use showed that VDR FokI-AA was associated with a lower risk of RA (p = 0.0255; OR = 0.58; 95% CI = 0.35–0.92, Table II). No other genetic polymorphism showed an association with RA in any of the models tested. Haplotype analysis adjusted by gender, age and tobacco use revealed that the haplotypes ACGAG (p = 0.033; OR = 1.62; 95% CI = 1.04–2.53) and GTGCA (p < 0.01; OR = 2.77; 95% CI = 1.53–4.98) for BsmI (rs1544410), Cdx2 (rs11568820), FokI (rs2228570), ApaI (rs7975232) and TaqI (rs731236) were associated with higher risk of RA (Table III).
Table II
Influence of clinical characteristics and VDR FokI rs2228570 gene polymorphism on risk of rheumatoid arthritis
Table III
Haplotype association with response adjusted by gender, age and tobacco use
Discussion
Rheumatoid arthritis is a complex disease in terms of its pathogenesis, since many factors influence its onset and development [35]. However, it has been found to have a significant and complex genetic component that plays a part in both the onset and clinical progression of the disease [35–37]. One of the genes most frequently studied due to its link with autoimmune diseases is the vitamin D receptor gene (VDR), the polymorphisms of which appear to contribute to bone resorption [13]. Rheumatoid arthritis is an autoimmune disease with pronounced inflammatory activity, in which Th1 and Th17 cells are involved, in addition to a high number of cytokines [38]. Different studies have found a relationship between VDR activity and the appearance, differentiation and function of T cells [39]. VDR gene polymorphisms can modify their activity and therefore the biological function of vitamin D, with an effect on the onset and progression of RA [23, 24]. Low levels of vitamin D can also play a part in disease progression [40]. A study performed on RA patients showed that lower levels of 1,25(OH)2D led to higher disease activity rates, measured using the DAS28 [41].
In terms of susceptibility to RA, the VDR gene FokI (rs2228570) polymorphism is one of the most widely studied [26, 42–47]. This polymorphism gives rise to a transcription start codon. The FokI-A variant produces a full (427 aa) VDR, whilst the VDR-FokI-G variant produces a shorter form of the protein (424 aa) and can increase the risk of developing RA [36]. In our study, the recessive model adjusted by gender, age and tobacco use showed a link between the AA genotype and a lower risk of RA in our patients (Table II), although the genotypic distribution of the FokI and BsmI polymorphisms in cases and controls does not fulfil the HWE. However, minor allele frequency in our population (A = 0.3271) is in consonance with the finding of the 1000 genomes for Iberian population in Spain (A = 0.369), so this indicated that we do not have a genotyping error. These results are consistent with a previous meta-analysis of 7 studies carried out on Caucasian and Asiatic populations (1156 cases/1494 controls), which revealed a higher risk of RA among individuals with at least one G allele of the VDR gene FokI polymorphism for the allelic model (G vs. A) [48]. In vitro studies have been carried out which show that the short version of the VDR gene protein has high transcriptional activity, which could cause Th1/Th2 imbalance and trigger the RA autoimmune process [44]. Another study by Monticielo et al. showed increased vitamin D levels in patients with the AA genotype compared with patients with the GG genotype [49], matching our results. Another in vitro study found that in GG genotype cells the VDR gene was over-expressed due to an increased transcription rate [50]. This phenomenon could trigger stimulation of the RA immune response as a result of the Th1/Th2 imbalance, since VDR has been demonstrated to be involved in the expression of inflammatory and immune response molecules [45].
No significant results were found in our study for the BsmI, TaqI and ApaI polymorphisms and risk of RA. BsmI and TaqI polymorphisms are found to be in linkage disequilibrium and are therefore inherited together, whilst FokI is not associated with any other VDR gene polymorphism and is inherited separately [45, 51], which might explain our results. Consistent with our study, a number of previous studies on Caucasian subjects found no association between these polymorphisms and RA [26, 44, 45, 52, 53]. In a study carried out on an Asiatic population, with 130 cases and 146 controls, an association was found between the Bsml polymorphism and risk of RA [13]. A study on lupus erythematosus, also carried out on an Asiatic population, found lower levels of mRNA in patients with the Bsml-G allele [37, 54]. This leads us to think that geographical location and race are factors that influence the relationship between the VDR gene and development of RA. However, in a meta-analysis of 7 studies with a total of 1152 cases and 880 controls, it was found that the TaqI-CC genotype may be a risk factor for RA in Caucasian European subjects [48], which suggests to us that we should increase the sample size of our study in order to improve the results obtained and find a relationship between VDR and RA. The link between VDR gene ApaI (rs7975232) polymorphism and risk of RA has been less widely studied. A study carried out on an Egyptian population, with 128 cases and 150 controls, found a significant difference between cases and controls as regards frequency of the VDR ApaI-CC genotype, which suggests that this genotype may play a part in risk of RA [46].
No other study to date has assessed the link between the VDR Cdx2 polymorphism and RA. We found no significant result in our study as regards this polymorphism. Some studies have described the association of the Cdx2 polymorphism with serum levels of vitamin D and the risk of fracture in osteoporosis patients [55]. Consistent with these results, the Cdx2-CC genotype has been identified as a risk factor in bone mineral metabolism and linear growth [29, 56].
The main limitation of this study is the small sample size, particularly as regards cases. This may have hindered the detection of associations for some polymorphisms. However, in spite of the limited sample size, and having performed a logistic regression analysis to prevent false positive associations, the effect of the VDR gene FokI (rs731236) polymorphism on risk of RA persisted. Further studies will be required with a broader range of genetic polymorphisms and a higher number of patients to definitively rule out the influence of these genes on the risk of RA. Among the strengths of our study is the highly homogeneous cohort of cases, since all cases of RA were diagnosed by the same team of rheumatologists and recruited in the same geographical area, which increases uniformity.
In conclusion, VDR FokI (rs2228570) gene polymorphism showed a trend for risk of RA, taking into account the variables of gender, age and tobacco use, and preventing false positives. Among our patients, VDR BsmI (rs1544410), TaqI (rs731236), ApaI (rs7975232) and Cdx2 (rs11568820) gene polymorphisms were not found to influence the risk of developing RA. However, haplotype analysis indicated that the haplotypes ACGAG and GTGCA were associated with higher risk of RA.