Introduction
Many induction regimens have been developed for the treatment of adult patients with acute lymphoblastic leukemia (ALL). The hyper-CVAD regimen (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone) has become a popular therapy for adult ALL patients. An effective dose-intensive regimen for adult patients with ALL was developed at the M.D. Anderson Cancer Center in the United States, and involves alternating hyper-CVAD with a high-dose MTX-Ara-C (methotrexate and cytarabine) regimen [1]. The treatment response to this regimen was good, with a complete remission rate of 92% and an estimated 5-year overall survival of 38% [2].
Although the remission rates with induction chemotherapy are good, most adult patients with ALL will finally relapse. Hemopoietic stem cell transplant (HSCT) is often performed to reduce the risk of relapse, especially in patients with high-risk ALL.
Although hyper-CVAD is generally associated with predictable and manageable toxicities, this regimen has the potential to reactivate viral infections such as hepatitis B virus (HBV) in chronically infected patients. Globally about 350 million people have chronic infection with HBV. The course of HBV reactivation in cancer patients is variable and may range from asymptomatic to fulminant hepatitis and even death. Fatal cases of HBV reactivation have been reported in patients with hematological malignancies including lymphoma and leukemia [3–6]. Hepatitis flare can also cause interruption of chemotherapy or a dose reduction, which may affect the efficacy of the chemotherapy. Clinicians initiating chemotherapy in hematology patients should be aware of the potential for hepatitis flare, which is a severe but preventable complication. There is limited published information on the carrier rate of hepatitis B in ALL and hepatitis B flare due to HBV reactivation in ALL patients. It is especially an important issue in HBV endemic areas, such as Asia.
We studied the outcomes of ALL patients treated with the hyper-CVAD regimen. We also investigated the carrier rate of HBV and risk of hepatitis flare due to HBV reactivation after chemotherapy. The results from our study will add information to the literature on these two issues.
Material and methods
Patient selection
This was a retrospective analysis of clinical records for patients aged 18 or above, with untreated ALL who had received hyper-CVAD chemotherapy between January 2005 and December 2016 in Tuen Mun Hospital in Hong Kong. Baseline parameters such as age at diagnosis, sex, clinical presentations of leukemia, central nervous system (CNS) involvement at diagnosis, the presence of any large mediastinal masses, complete blood profile and bone marrow results were collected.
ALL diagnosis was confirmed using bone marrow aspirate and trephine morphology, immunophenotype, and cytogenetic results. Patients were referred for allogenic stem cell transplant if a suitable donor was found and the disease was in remission. The complete remission rate, overall survival and toxicity of the hyper-CVAD regimen were analyzed.
Viral screening of blood samples included hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (anti-HBs) as well as hepatitis C virus antibody (anti-HCV) and human immunodeficiency virus (HIV). Liver biochemistry was monitored regularly before and after starting chemotherapy. Before 2010, we did not routinely screen for the presence of antibody to hepatitis B core antigen (anti-HBc) in ALL patients, but anti-HBc testing was added to the screening protocol from 2010 onwards after the report of HBV reactivation in patients with a hematological malignancy and anti-HBc [7].
We defined the HBV-infected patients as those with detectable levels of HBsAg when ALL was diagnosed. Hepatitis flare due to HBV reactivation was defined as a 10-fold rise in HBV deoxyribonucleic acid (HBV-DNA) and 3-fold rise in serum alanine aminotransferase (ALT) compared with baseline. For anti-HBc positive patients, HBV reactivation was defined as seroconversion from HBV DNA negative to positive.
In patients who developed hepatitis, the severity was classified according to the criteria of the World Health Organization (WHO). Grade 1 was defined as ALT elevated to > 1.25× the upper limit of normal (ULN) and up to 2.5× ULN; grade 2 was ALT elevated to > 2.5× ULN and up to 5.0× ULN; grade 3 was an elevation of ALT > 5.0× ULN and up to 10.0× ULN; and grade 4 was an elevation of ALT > 10× ULN [8].
Treatment
The chemotherapy regimen included a dose-intensive phase in which cycles of hyper-CVAD were alternated with high-dose intravenous methotrexate (MTX) and cytarabine, up to a maximum of 8 cycles, followed by 2 years of maintenance therapy for patients not undergoing HSCT. The details of the protocol have been described previously [1, 2].
Patients with Philadelphia chromosome (Ph) positive ALL also received a tyrosine kinase inhibitor concurrently with hyper-CVAD chemotherapy during induction, consolidation and maintenance therapy. Patients with high-risk disease (complex cytogenetics, high initial white blood cell (WBC) count) and a matched donor underwent allogenic HSCT. If there was no matched donor, they received maintenance therapy for 2 years. Individual patients who had a matched family donor but did not have high-risk features were also considered for HSCT.
CNS prophylaxis consisted of intrathecal administrations of MTX 12 mg on day 2 and cytarabine 100 mg on day 7 of each cycle. All patients received standard antimicrobial prophylaxis consisting of oral levofloxacin, itraconazole and acyclovir starting from the beginning of chemotherapy. They also received trimethoprim/sulfamethoxazole for prevention of Pneumocystis jiroveci pneumonia. Patients who were positive for HBsAg or anti-HBc were given specific antiviral prophylaxis for HBV reactivation from the beginning of chemotherapy until at least 1 year after stopping chemotherapy. The antiviral prophylaxis was lamivudine 100 mg daily from 2005 to 2013, but was changed to entecavir 0.5 mg daily in 2014 because of growing evidence that entecavir was a more effective option than lamivudine for preventing HBV reactivation [9].
Response assessment and statistical analysis
We performed bone marrow examination at the end of the induction phase of chemotherapy. If complete remission (CR) could not be achieved, bone marrow examination was repeated at the end of each cycle of therapy until CR was documented. CR was defined as less than 5% blasts on bone marrow morphologic examination as well as hematologic recovery.
The primary outcomes were overall survival and CR rate. A univariate analysis of risk factors for decreased overall survival or death was performed by calculating the hazard ratio (HR) and 95% confidence intervals (CI). The prognostic significance of age, sex, WBC, Ph chromosome, HBV status and bulky disease was assessed. This was followed by a Cox proportional hazards model using variables with a significant impact on outcomes in the univariate analysis (p < 0.05). We calculated the overall survival from the date of ALL diagnosis until death or censored at the last follow-up date, and analyzed using Kaplan-Meier survival analysis and the log-rank test. The p-values were 2-sided and p-values of < 0.05 were defined as statistically significant.
Results
Patient characteristics
A total of 52 ALL patients were treated with hyper-CVAD in the study period (Table I). The median age at diagnosis was 42 years (range: 20–74). The male to female ratio was 1.36 (30/22). The median follow-up time was 72 months (1.0–149.1). No patient had CNS disease at diagnosis. Forty-one patients had B-cell ALL (79%). The median WBC was 11.35 (0.4–178) × 109/l, median hemoglobin was 9.6 (3.5–14.6) g/dl, and median platelet count was 56 (7–429) × 109/l. Thirteen (25%) patients had a WBC more than 30 × 109 cells/l at diagnosis.
Table I
Characteristics of ALL patients in our study
Diagnostic cytogenetic results were available in 51 (95%) patients, but no bone marrow aspirate could be obtained (dry tap) in one patient. Fourteen (27%) patients were Ph-positive, and were given tyrosine kinase inhibitor together with hyper-CVAD chemotherapy.
Treatment response
Forty-seven (90.4%) patients achieved CR after the first cycle of induction. Three patients required two cycles of hyper-CVAD to achieve CR. One had primary refractory disease. Another patient died of infection during induction chemotherapy. The median overall survival was 39.6 months and the 5-year survival rate was 50% (Figure 1). Bone marrow relapse occurred in 21 patients. The median time from CR to relapse was 9 months (3–66 months). There was no CNS relapse. One (1.9%) patient died during induction. This patient was 58 years old, and died of pneumonia and Escherichia coli septicemia about 1 month after his ALL was diagnosed.
Figure 1
Overall survival of acute lymphoblastic leukemia (ALL) patients treated with hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone)
Overall, 31 (59.6%) patients underwent allogenic HSCT, of whom 14 were Ph-positive. Twelve of the 31 patients relapsed after HSCT, and only one of these patients achieved further CR after post-HSCT relapse.
Prognostic factors
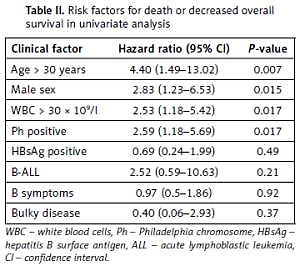
Risk factors for reduced overall survival on univariate analysis included WBC > 30 × 109 cells/l (hazard ratio (HR) = 2.53, 95% confidence interval (CI): 1.18–5.422, p = 0.017), age > 30 years (HR = 4.40, 95% CI: 1.49–13.02, p = 0.007), male sex (HR = 2.83, 95% CI: 1.23–6.53, p = 0.015), and presence of Ph chromosome (HR = 2.59, 95% CI: 1.18–5.69, p = 0.017). HBsAg status was not significantly associated with overall survival (HR = 0.69, 95% CI: 0.24–1.99, p = 0.49) (Table II). In the multivariate analysis using a Cox proportional model, WBC more than 30 × 109 cells/l (HR = 2.52, 95% CI: 1.11–5.75, p = 0.028) and age > 30 years (HR = 3.56, 95% CI: 1.14–11.16, p = 0.029) remained significant prognostic factors for reduced overall survival.
Table II
Risk factors for death or decreased overall survival in univariate analysis
Hepatitis flares in ALL patients
Nine (17.3%) patients were HBsAg positive at the time of ALL diagnosis. Of the 28 (14%) patients who were tested for anti-HBc, four were anti-HBc positive. These 13 patients were given antiviral prophylaxis from the beginning of chemotherapy until at least 12 months after finishing chemotherapy.
One (11%) HBsAg-positive patient developed a hepatitis flare due to HBV reactivation., at about 12 months after starting chemotherapy. He was on lamivudine prophylaxis, and the peak HBV-DNA was 4.43 × 106 IU/ml. The highest ALT level was 1061 U/l. Ultrasound of the abdomen was unremarkable. Antiviral therapy was changed to entecavir after the hepatitis flare. Liver enzymes gradually normalized and the patient could continue the chemotherapy. HBV-DNA was not detected later.
Toxicity
One patient died of pneumonia during induction chemotherapy. Three patients died of infective complications (pneumonia) during hyper-CVAD chemotherapy. These patients ranged in age from 21 to 67 years. Two of these patients had relapsed leukemia.
Three patients developed perianal abscesses, but these responded to antibiotic treatment and surgical drainage of the abscess. One patient had CNS infection. None of the patients in our cohort developed thrombosis, and there was no cerebellar toxicity resulting from high-dose cytarabine.
Discussion
The hyper-CVAD regimen was developed at the M.D. Anderson Cancer Center for the treatment of ALL and high-grade lymphoma patients [10]. The outcomes of the hyper-CVAD regimen have been shown to be significantly superior to outcomes with the VAD (vincristine, doxorubicin and dexamethasone) regimen [1, 2], with a CR rate of 91% and a 5-year survival rate of 39% [1, 2]. Hyper-CVAD is now one of the most frequently used chemotherapeutic regimens for the treatment of adult ALL.
Since the introduction of hyper-CVAD for ALL at our institution, 52 patients have been treated (median age: 42 years), and this retrospective analysis showed that the CR rate in this real-world cohort of ALL patients receiving hyper-CVAD was 90%. The induction mortality rate was 1.8%. The median survival was 39.6 months and the 5-year survival rate was 50%. Three patients in our study died of pneumonia. Two of them also had relapsed leukemia, which could have contributed to the mortality.
Our results compare favorably with the results of other published studies (Table III) [1, 2, 11–16]. The overall survival in our study was slightly higher than some of the previously published studies. It could be due to more HSCT in high-risk patients and adequate antibiotic prophylaxis in our study. Patients in some studies might not receive adequate anti-infective prophylaxis [13–15]. For example, trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis jiroveci was not applied in all study institutions in one study [14].
Table III
Comparison of results of hyper-CVAD in ALL patients in different studies
| Reference | N | Median age [years] | Ph positive | CR | Induction mortality rate | Median survival [months] | Overall survival |
|---|---|---|---|---|---|---|---|
| Kantarjian et al. 2000 [1] | 204 | 39.5 | 17% | 91% | 5% | 35 | 39% at 5 years |
| Kantarjian et al. 2004 [2] | 288 | 40 | 17% | 92% | 5% | 32 | 38% at 5 years |
| Xu et al. 2008 [11] | 53 | 30 | 11.3% | 73.6% | 0% | 48.7 | 83% at 2 years |
| Morris et al. 2011 [12] | 63 | 29 | 7% | 86% | 5% | 54 | 48% at 5 years |
| Abbasi et al. 2013 [13] | 66 | 32.9 | 16% | 90% | 5% | 30 | 20% at 5 years |
| Buyukasik et al. 2013 [14] | 57 | 29 | 12% | 84.2% | 10.5% | 16.4 | 26.3% at 5 years |
| Alacacioglu et al. 2014 [15] | 30 | 30.5 | 13.3% | 96% | N/A | 41.5 | 34% at 5 years |
| Portugal et al. 2015 [16] | 49 | 30 | 13.6% | 93.8% | 4% | 24.4 | 35% at 5 years |
| Present study | 52 | 42 | 27% | 90.4% | 1.8% | 39.6 | 50% at 5 years |
In our experience, hyper-CVAD was easy to administer. Some patients could be discharged from hospital soon after finishing chemotherapy. The regimen was tolerable and the toxicity was acceptable. Compared with other studies, a higher proportion of ALL patients in our cohort underwent HSCT. HSCT is an attractive option for the treatment of ALL, especially in younger patients.
Previous analyses have shown that advanced age, high WBC count, and presence of the Ph chromosome are poor prognostic factors in ALL [17]. Results in our cohort also showed that WBC > 30 × 109 cells/l and age > 30 years predict reduced survival after hyper-CVAD in ALL.
In addition to markers of risk such as WBC and age, there is increasing evidence that biomarkers of cellular apoptosis and autophagy, such as beclin-1 and B-cell lymphoma-2 (Bcl-2) levels, may have potential prognostic value in ALL [18]. Autophagy is a catabolic pathway involving degradation of lysosomes and then recycling of cell proteins and organelles [19]. Abdelsalam et al. studied the expression levels of a series of autophagic beclin-1, pro-apoptotic (Bad and Bax), and anti-apoptotic (Bcl-2 and Bcl-xL) parameters, illustrating the correlation between their levels in ALL. There was a positive correlation with Bcl-2, while there was a negative correlation with beclin-1 [18].
Ph positive ALL
Our patients received a tyrosine kinase inhibitor (TKI) in combination with hyper-CVAD chemotherapy if they were Ph positive. They also received HSCT if a suitable donor was available. The incorporation of TKI into hyper-CVAD chemotherapy in the treatment of Ph-positive ALL has improved the outcome of these patients, especially when the TKIs are combined early and continuously with chemotherapy [20–22]. Therefore, TKIs in combination with chemotherapy are the standard of care for all patients with Ph-positive ALL.
Second-generation TKIs appear to be more potent than imatinib [23–25]. Dasatinib also inhibits SRC kinases, which are involved in the pathophysiology of Ph-positive ALL [26]. Hyper-CVAD in combination with dasatinib has shown encouraging results in ALL patients, with a CR rate of 96%, and median overall survival of 47 months [27]. Another study comparing ponatinib with dasatinib in combination with hyper-CVAD showed an even better survival benefit in the ponatinib group. The 3-year overall survival rates were 83% and 56% in the ponatinib and dasatinib groups, respectively [28]. Despite the improved responses with TKIs in Ph-positive ALL, allogenic HSCT remains the standard of treatment [29, 30].
HBV reactivation in ALL patients with HBV infection
The HBV carrier rate was 17.3% in our cohort, which is higher than in the general population in Hong Kong [31]. There is evidence that HBV infection is more common in patients with lymphoma [32, 33]. It has been postulated that HBV can integrate into the host genome, where it can downregulate the expression of tumor suppression genes or increase expression of cellular oncogenes [34, 35]. Alternatively, the persistent antigenic stimulation and B-lymphocyte proliferation that occur during chronic HBV infection may predispose patients to genetic aberrations or double-stranded DNA breaks that could lead to malignant transformation and also proliferation.
Most of the data on HBV reactivation in cancer patients are from lymphoma patients being given chemotherapy and rituximab. There are few data on acute leukemia, and the correlation between HBV infection and ALL is not yet known. In a previous study in patients with acute myeloid leukemia HBV reactivation occurred in 13% of HBsAg positive patients who received antiviral prophylaxis during chemotherapy compared with 61% of patients who did not receive antiviral prophylaxis [3].
Univariate analysis in our cohort did not find HBV infection as a significant predictor of reduced overall survival. Our data suggest that patients who are HBsAg positive can have similar survival as HBsAg negative patients if they are given adequate antiviral prophylaxis. Therefore, approaches to prevent HBV reactivation are clinically important in HBV infected ALL patients undergoing chemotherapy.
HBV reactivation depends on the cancer type, host factors and types of therapy. HBV reactivation is particularly common in patients receiving chemotherapy for hematological malignancies, such as acute leukemia, because of the intensive chemotherapy regimens. The risk of HBV reactivation in patients receiving chemotherapy is a particular problem in endemic areas, especially among those who are HbsAg-positive. Rituximab is known to increase the risk of HBV reactivation, and a preventive strategy for HBV reactivation is required when rituximab is used. Rituximab was found to be useful in B-ALL [36]. A prospective study showed that rituximab in combination with an ALL chemotherapy protocol improved outcomes, including event-free survival, in younger adults with CD20-positive, Ph-negative ALL. There was longer event-free survival in the rituximab group than in the control group (65% vs. 52%) after a median follow-up of 30 months (HR = 0.66; p = 0.04), attributed to lower rates of relapse of ALL [36].
HBV reactivation may be an exacerbation of a chronic hepatitis B infection or recurrence of a previous infection. There are two major types of patients: those with a current infection (defined by the presence of HBsAg), and those with occult HBV infection (defined by the presence of anti-HBc antibody). Patients with anti-HBc without HBsAg are considered to have resolved HBV infection, but they are still at risk of HBV reactivation and a serious or even fatal outcome during chemotherapy, particularly if receiving rituximab. Once the HBV infection has resolved, HBV DNA is rarely detected in peripheral blood, but trace amounts are often present in the liver, where the virus can be activated during immunosuppression [37].
Prophylactic antiviral therapy should be given to patients who are positive for HBsAg to prevent HBV reactivation. They should be given prophylactic antiviral treatment before initiation of chemotherapy or concurrently with chemotherapy, regardless of the level of HBV DNA. Antiviral prophylaxis is highly effective in preventing HBV reaction, with one reactivation prevented for every 3 patients treated [38]. However, a pre-emptive strategy, in which antiviral therapy is initiated once HBV reactivation is detected, is much less effective than prophylactic antiviral therapy in preventing hepatitis flares in HBsAg-positive patients [7, 39, 40]. This is because of the time required for antiviral therapy to reduce viral load and control the disease (usually weeks to months), during which hepatic inflammation and necrosis can persist [41].
There is no standard strategy for the prevention of HBV reactivation in ALL patients with resolved HBV infection, and two possible strategies are available. One is to monitor HBV DNA regularly during treatment and initiate pre-emptive antiviral therapy as soon as HBV DNA becomes detectable. However, there is no consensus on the optimal interval of monitoring [39, 42], and HBV DNA monitoring is costly.
The other strategy is prophylactic antiviral therapy, which may be the preferred option during high-risk chemotherapy such as rituximab and anthracycline-based chemotherapy, and those without anti-HBs. This approach can effectively prevent HBV reactivation without the inconvenience and cost of repeated HBV DNA monitoring [43]. Another potential advantage is that patients receive antiviral therapy early when there is minimal viremia, which is likely to optimize the efficacy of treatment, since antiviral agents tend to be less effective in patients with high viral titers.
We adopted a protocol of prophylactic antiviral therapy to prevent hepatitis flare due to HBV reactivation in our ALL patients receiving doxorubicin as part of a hyper-CVAD regimen based on data showing that prophylaxis is more effective than pre-emptive antiviral therapy in high-risk patients. Hyper-CVAD contains doxorubicin, which along with other anthracycline derivatives is considered to carry a moderate risk of HBV reactivation (1–10% risk) in patients with resolved HBV infection in the guideline published by the American College of Gastroenterology [44].
Another issue is the choice of antiviral prophylaxis. As the first oral antiviral agent to be used for the treatment of chronic HBV infection, lamivudine has been the most studied prophylactic agent. In randomized trials, lamivudine has proven to be effective in preventing HBV reactivation in patients receiving immunosuppressive therapies [40], and is well tolerated and safe. However, viral resistance can develop when lamivudine is used for more than 6 months because of the emergence of tyrosine-methionine-aspartic acid-aspartic acid (YMDD) mutations. As a result, newer agents such as entecavir or tenofovir, which are associated with a lower rate of resistance, are now preferred to lamivudine for the treatment of chronic HBV infection. In a prospective randomized study comparing lamivudine and entecavir in patients undergoing chemotherapy, the rate of HBV reactivation (30% vs. 6.6%, p = 0.001), HBV-related hepatitis (13.3% vs. 0%, p = 0.003), and chemotherapy disruption (18.3% vs. 1.6%, p = 0.002) were all significantly higher in the lamivudine than the entecavir group [9]. One of our patients on lamivudine prophylaxis developed hepatitis flare, indicating that lamivudine had been less effective in preventing HBV reactivation. Entecavir or tenofovir would be a better choice of antiviral prophylaxis. If the patient has renal impairment, entecavir would be more suitable as the choice of antiviral prophylaxis.
Limitations of our study include its retrospective design and relatively small sample size. In addition, some patients with profound thrombocytopenia did not undergo lumbar puncture at the time of initial diagnosis because of the bleeding risk, so we cannot rule out the possibility that some of these patients had CNS disease at baseline. Similarly, not all patients in our cohort were tested for anti-HBc because this test was not introduced as part of our routine viral testing protocol for ALL patients until 2010.
In conclusion, our study showed that hyper-CVAD is effective, tolerable and easy to administer in adult ALL patients. It is an option for the treatment of ALL. The risk of hepatitis flare due to HBV reactivation can be reduced by administering antiviral prophylaxis in patients with HBV infection.