Introduction
Dermatomyositis (DM) is a systemic autoimmune disease primarily affecting muscles, skin, and lungs [1]. Anti-melanoma differentiation-associated gene 5-positive dermatomyositis (anti-MDA5+ DM) is a specific type of DM, characterized by unique skin rashes, rapidly progressive interstitial lung disease (RPILD), peripheral lymphocytopenia, and elevated serum ferritin levels, with a poor prognosis [2]. Antinuclear antibodies (ANAs) are a group of autoantibodies that target nuclear components such as nuclear proteins and nucleic acids in the cell nucleus or cytoplasm of eukaryotic cells [3]. ANAs are commonly found in patients with rheumatic diseases, as well as in non-rheumatic autoimmune diseases, infections, malignant tumors, and normal individuals. ANAs exhibit different titers and staining patterns [4]. A positive ANA test has limited predictive value for the diagnosis of connective tissue diseases (CTD). However, a negative result does not rule out the presence of challenging rheumatic diseases in clinical practice. Recent studies have shown that indicators including myositis-specific antibodies, clinical features, and peripheral lymphocyte count may help classify patients with DM into different prognostic subgroups [5, 6]. Antinuclear antibody (ANA) testing is commonly used in suspected DM patients, with 50–78% of confirmed cases testing positive [7, 8]. It has been observed that anti-MDA5+ DM patients frequently have positive ANA [9]. However, the clinical significance of ANA status in anti-MDA5+ DM patients has not been clearly defined. This study aims to explore the effects of ANA on the clinical characteristics and prognosis of anti-MDA5+ DM.
Material and methods
We conducted a retrospective cohort study on patients from the Nanjing Medical University Myositis-Associated Interstitial Lung Disease (NMMI) cohort. The NMMI cohort is a multicenter, retrospective, longitudinal cohort comprising data from ten tertiary hospitals in the East China region. This cohort has been previously described in detail and used in multiple studies [10–12].
The diagnosis of DM was based on the 1975 Bohan/Peter Criteria or Sontheimer’s Criteria or the 1996 American Academy of Dermatology Dermatomyositis Care Guidelines [13–15]. The diagnosis of interstitial lung disease (ILD) and rapidly progressive interstitial lung disease (RPILD) was based on internationally accepted standards [16, 17]. The specific details of the diagnosis and assessment of ILD and RPILD in this cohort study have been described in detail in previous studies [10, 11]. Patients with other autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, gout, ankylosing spondylitis, IgG4-related disease, systemic sclerosis, and those with missing or incomplete clinical data were excluded from this study. The study endpoint is defined as the occurrence of death. All patients were followed up for 12 months, and if a patient died during the 1-year follow-up period, it was recorded as an adverse outcome endpoint. Overall survival is defined as the time from the initial diagnosis to the date of death or the last follow-up for anti-MDA5+ DM patients.
This study included 246 anti-MDA5+ DM patients and collected general demographic, clinical, and laboratory data for research purposes. The variables in the study include three aspects: (i) general demographic characteristics (gender, age, disease duration, follow-up period); (ii) clinical characteristics of anti-MDA5+ DM – proximal muscle involvement (it denotes proximal muscle weakness), rash, Gottron papule, heliotrope rash, V sign, shawl sign, periungual erythema, arthritis, mechanic’s hands, and cutaneous ulcers; (iii) laboratory tests – alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum ferritin, ANA, anti-Ro52 antibody, and anti-aminoacyl-tRNA synthetase (anti-ARS) antibody. The detection of ANA was tested by indirect immunofluorescence assays (IIFAs) on HEp-2 cells. Anti-Ro52 antibodies, anti-ARS antibodies, and anti-MDA5 were measured by immunoblotting, with anti-MDA5 titers categorized into three groups (MDA5+, MDA5++, MDA5+++).
The study protocol was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University (Ethics Approval No: JD-HG-2023-09). Due to the retrospective nature of the study and the anonymity of the data, the requirement for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
The data in this study were analyzed using SPSS statistical software version 25.0 (IBM Corporation, New York, USA) and R software (version 4.2.1). For continuous variables, the Kolmogorov-Smirnov test and Levene’s test were first conducted. Normally distributed continuous variables were presented as mean (standard deviation, SD) and compared between groups using Student’s t-test. Skewed distributed continuous variables were presented as the median and interquartile range (P25, P75) and compared between groups using the Mann-Whitney U test. For categorical variables, frequencies (percentages) were presented, and differences between groups were tested using the χ2 test or Fisher’s exact test. Overall survival time was calculated from the date of initial diagnosis to either death or the end of the study. Univariate and multivariate Cox regression models were created to identify factors associated with mortality for anti-MDA5+ DM, presented as hazard ratios (HR) and 95% confidence intervals (CI). Survival analysis was performed using the Kaplan-Meier method, and differences in mortality rates between two groups of patients were compared using the log-rank test. All analyses were 2-tailed and p-values < 0.05 were considered to indicate statistical significance. The comparison between different anti-MDA5 antibody titer groups was performed using the independent sample’s Kruskal-Wallis test.
Results
This study recruited 246 anti-MDA5+ DM patients, with 28.5% male and 71.5% female. The median age was 53.0 years, the median duration of disease was 2 months, and the median follow-up period was 12.0 months. In terms of clinical manifestations, 93.1% of patients had skin rashes, 68.3% had Gottron papules, 56.9% had heliotrope rash, 36.2% had V sign, 13.8% had cutaneous ulcers, and 45.5% had proximal muscle involvement. In laboratory tests, the median ALT was 47.3 (29.0, 80.5) U/l, AST was 52.0 (32.9, 83.0) U/l, LDH was 421.1 ±311.8 U/l, CK was 63.0 (36.8, 158.0) U/l, CRP was 5.9 (3.1, 12.2) mg/l, and serum ferritin median was 1307.6 ±2363.3 ng/ml. 64.2% of patients were positive for anti-Ro52 antibody, and 6.1% were positive for anti-ARS antibody. The distribution of anti-MDA5 antibody titers was 29.3% +, 18.7% ++, and 52.0% +++. All patients had ILD, 35.8% had RPILD, and the overall mortality rate was 24.4%. The anti-MDA5+ DM patients were then divided into two groups based on whether their ANA was positive. There were no significant differences between ANA+ and ANA- patients in terms of gender, age, duration of disease, clinical characteristics, and mortality rates. Compared to the ANA- group, ANA+ patients had higher rates of positivity for anti-Ro52 and anti-ARS antibody, and lower ALT levels (p < 0.05) (Table I).
Table I
Baseline characteristics of anti-MDA5+ DM patients with positive and negative ANA
| Variable | Total (n = 246) | ANA+ (n = 129) | ANA– (n = 117) | P-value |
|---|---|---|---|---|
| Gender, n (%) | 0.629 | |||
| Male | 70 (28.5) | 35 (27.1) | 35 (29.9) | |
| Female | 176 (71.5) | 94 (72.9) | 82 (70.1) | |
| Age, median (range) [years] | 53.0 (47.0, 63.0) | 53.0 (47.0, 63.0) | 53.0 (45.0, 62.5) | 0.745 |
| Course of the disease, median (range) [months] | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 2.0 (1.0, 6.0) | 0.201 |
| Follow-up periods, median (range) [months] | 12.0 (3.0, 12.0) | 12.0 (3.0, 12.0) | 10.0 (3.0, 12.0) | 0.524 |
| Proximal muscle involvement#, n (%) | 112 (45.5) | 57 (44.2) | 55 (47.0) | 0.657 |
| Rash, n (%) | 229 (93.1) | 118 (91.5) | 111 (94.9) | 0.294 |
| Gottron papule, n (%) | 168 (68.3) | 86 (66.7) | 82 (70.1) | 0.565 |
| Heliotrope rash, n (%) | 140 (56.9) | 73 (56.6) | 67 (57.3) | 0.915 |
| V sign, n (%) | 89 (36.2) | 50 (38.8) | 39 (33.3) | 0.376 |
| Cutaneous ulcers, n (%) | 34 (13.8) | 15 (11.6) | 19 (16.2) | 0.295 |
| Shawl sign, n (%) | 55 (22.4) | 28 (21.7) | 27 (23.1) | 0.797 |
| Periungual erythema, n (%) | 52 (21.1) | 33 (25.6) | 19 (16.2) | 0.073 |
| Arthritis, n (%) | 90 (36.6) | 49 (38.0) | 41 (35.0) | 0.632 |
| Mechanic’s hands, n (%) | 67 (27.2) | 36 (27.9) | 31 (26.5) | 0.804 |
| ALT, median (range) [U/l] | 47.3 (29.0, 80.5) | 39.0 (21.5, 79.3) | 51.3 (36.5, 95.8) | 0.006* |
| AST, median (range) [U/l] | 52.0 (32.9, 83.0) | 50.0 (32.0, 83.0) | 52.2 (33.7, 84.5) | 0.437 |
| LDH, median (range) [U/l] | 421.1 ±311.8 | 340.0 (253.0, 434.5) | 337.0 (259.5, 422.6) | 0.754 |
| CK, median (range) [U/l] | 63.0 (36.8, 158.0) | 67.0 (36.0, 184.5) | 60.0 (37.0, 132.0) | 0.319 |
| ESR, median (range) [mm/h] | 42.0 ±23.8 | 41.0 (27.0, 58.5) | 37.0 (22.5, 51.5) | 0.085 |
| CRP, median (range) [mg/l] | 5.9 (3.1, 12.2) | 5.9 (3.1, 12.2) | 5.9 (3.1, 12.2) | 0.791 |
| Serum ferritin [ng/ml] | 1307.6 ±2363.3 | 1307.6 (579.5, 1394.3) | 1186.0 (462.5, 1307.6) | 0.452 |
| Anti-Ro52 positive, n (%) | 158 (64.2) | 94 (72.9) | 64 (54.7) | 0.003* |
| Anti-ARS positive, n (%) | 15 (6.1) | 12 (9.3) | 3 (2.6) | 0.033* |
| Anti-MDA5 antibody titer, n (%) | 0.553 | |||
| + | 72 (29.3) | 41 (31.8) | 31 (26.5) | - |
| ++ | 46 (18.7) | 25 (19.4) | 21 (17.9) | - |
| +++ | 128 (52.0) | 63 (48.8) | 65 (55.6) | - |
| ILD, n (%) | 246 (100.0) | 129 (100.0) | 117 (100.0) | - |
| RPILD, n (%) | 88 (35.8) | 50 (38.8) | 38 (32.5) | 0.305 |
| Death, n (%) | 60 (24.4) | 31 (24.0) | 29 (24.8) | 0.890 |
* Values statistically significant at p < 0.05. anti-MDA5+ DM – anti-melanoma differentiation-associated gene 5 positive dermatomyositis, RPILD – rapidly progressive interstitial lung disease, ALT – alanine aminotransferase, AST – aspartate aminotransferase, CK – creatine kinase, LDH – lactate dehydrogenase, ESR – erythrocyte sedimentation rate, CRP – C-reactive protein, ANA – antinuclear antibody, Anti-ARS – anti-aminoacyl-tRNA synthetase.
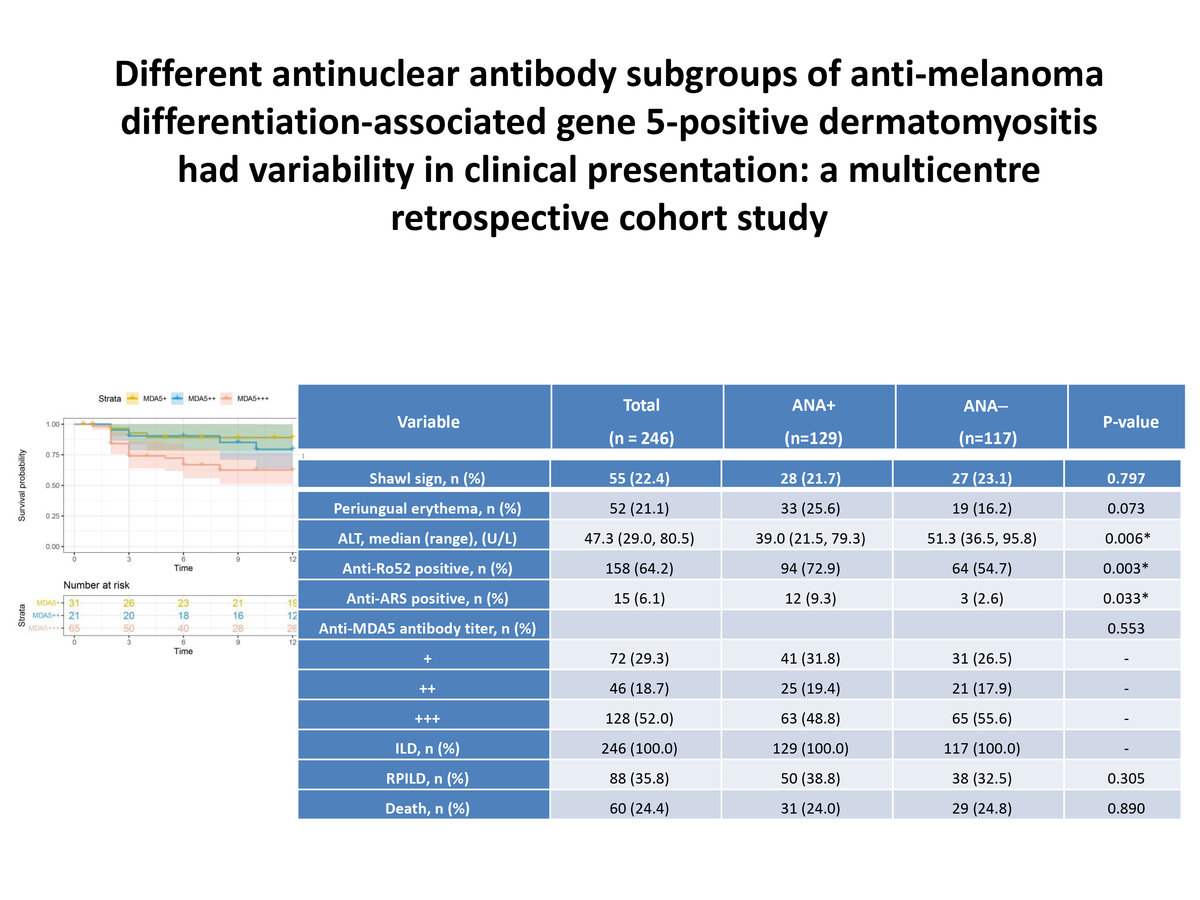
A total of 246 patients were followed up for a range of 0.5 to 12 months, with a median follow-up time of 12 months. After 12 months of follow-up, a total of 60 patients reached the endpoint event. Comparison of the survival time between anti-MDA5+ DM patients with positive and negative ANA showed no statistically significant difference in mortality rate (log-rank p = 0.890). The Kaplan-Meier curves are shown in Figure 1.
Figure 1
Survival analysis of anti-MDA5+ DM patients with positive and negative ANA. The survival analysis indicated no statistically significant difference in mortality rate between anti-MDA5+ DM patients with positive and negative ANA (log-rank p = 0.890)
We separately analyzed the clinical characteristics of 129 anti-MDA5+DM patients in the subgroups with positive ANA. Patients were divided into three groups based on different anti-MDA5 antibody titers, including 41 cases in the MDA5+ group, 25 cases in the MDA5++ group, and 63 cases in the MDA5+++ group. There were no significant differences among the three groups of patients in terms of gender, age, disease duration, clinical features, laboratory tests, and mortality rates. Regarding serum ferritin levels, the MDA5+++ group had higher levels compared to the MDA5++ group (p < 0.05). In terms of RPILD, the incidence rate was higher in MDA5+++ patients compared to the MDA5+ group (p < 0.05) (Table II).
Table II
Baseline characteristics of three groups of patients with different anti-MDA5 antibody titers among 129 ANA-positive patients
| Variable | ANA+/MDA5+ (n = 41) | ANA+/MDA5++ (n = 25) | ANA+/MDA5+++ (n = 63) | P-value |
|---|---|---|---|---|
| Male, n (%) | 6 (14.6) | 7 (28.0) | 22 (34.9) | 0.075 |
| Female, n (%) | 35 (85.4) | 18 (72.0) | 41 (65.1) | |
| Age, median (range) [years] | 53.0 (48.0, 61.5) | 54.0 (48.0, 64.5) | 52.0 (47.0, 63.0) | 0.866 |
| Course of the disease, median (range) [months] | 2.0 (1.0, 3.0) | 2.0 (1.0, 6.0) | 2.0 (1.0, 4.0) | 0.561 |
| Proximal muscle involvement#, n (%) | 16 (39.0) | 14 (56.0) | 27 (42.9) | 0.386 |
| Rash, n (%) | 36 (87.8) | 24 (96.0) | 58 (92.1) | 0.500 |
| Gottron papule, n (%) | 26 (63.4) | 18 (72.0) | 42 (66.7) | 0.773 |
| Heliotrope rash, n (%) | 23 (56.1) | 14 56.0) | 36 (57.1) | 0.992 |
| V sign, n (%) | 17 (41.5) | 9 (36.0) | 24 (38.1) | 0.897 |
| Cutaneous ulcers, n (%) | 3 (7.3) | 0 (0.0) | 12 (19.0) | - |
| Shawl sign, n (%) | 8 (19.5) | 5 (20.0) | 15 (23.8) | 0.851 |
| Periungual erythema, n (%) | 8 (19.5) | 3 (12.0) | 22 (34.9) | 0.052 |
| Arthritis, n (%) | 18 (43.9) | 8 (32.0) | 23 (36.5) | 0.592 |
| Mechanic’s hands, n (%) | 14 (34.1) | 7 (28.0) | 15 (23.8) | 0.517 |
| ALT, median (range) [U/l] | 37.8 (21.5, 79.3) | 33.0 (16.0, 93.0) | 46.0 (28.0, 79.6) | 0.419 |
| AST, median (range) [U/l] | 49.6 (27.2, 81.9) | 48.0 (25.0, 84.0) | 53.0 (34.0, 89.0) | 0.565 |
| LDH, median (range) [U/l] | 317.0 (246.5, 398.0) | 397.0 (258.5, 679.0) | 342.0 (259.0, 440.0) | 0.319 |
| CK, median (range) [U/l] | 48.0 (26.4, 139.0) | 93.0 (45.5, 176.5) | 69.0 (40.0, 221.0) | 0.151 |
| ESR, median (range) [mm/h] | 34.0 (26.9, 53.1) | 37.0 (18.5, 70.5) | 46.0 (27.0, 59.0) | 0.287 |
| CRP, median (range) [mg/l] | 4.7 (2.4, 10.9) | 5.6 (3.4,14.7) | 7.8 (3.1, 16.6) | 0.199 |
| Serum ferritin [ng/ml] | 1307.6 (570.2, 1307.6) | 711.2 (165.0, 1395.3)a | 1307.6 (908.7, 1500.0)a | 0.018* |
| Anti-Ro52 positive, n (%) | 30 (73.2) | 19 (76.0) | 45 (71.4) | 0.908 |
| Anti-ARS positive, n (%) | 4 (9.8) | 0 (0.0) | 8 (12.7) | 0.206 |
| ILD, n (%) | 41 (100.0) | 25 (100.0) | 63 (100.0) | – |
| RPILD, n (%) | 9 (22.0)a | 12 (48.0) | 29 (46.0)a | 0.028* |
| Death, n (%) | 5 (12.2) | 6 (24.0) | 20 (31.7) | 0.074 |
* Values statistically significant at p < 0.05. anti-MDA5+ DM – anti-melanoma differentiation-associated gene 5 positive dermatomyositis, RPILD – rapidly progressive interstitial lung disease, ALT – alanine aminotransferase, AST – aspartate aminotransferase, CK – creatine kinase, LDH – lactate dehydrogenase, ESR – erythrocyte sedimentation rate, CRP – C-reactive protein, ANA – antinuclear antibody, Anti-ARS – anti-aminoacyl-tRNA synthetase.
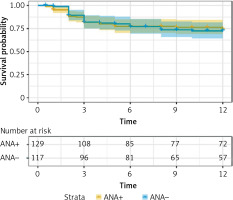
The follow-up period for 129 ANA-positive patients ranged from 0.5 months to 12 months, with a median follow-up time of 12 months. Thirty-one patients died during the follow-up period (1 year). The survival times of three groups of patients (ANA+/MDA5+; ANA+/MDA5++; ANA+/MDA5+++) were compared. The results showed that the mortality rates of the three patient groups with different anti-MDA5 antibody titers were not significantly different (log-rank p = 0.085). The Kaplan-Meier curves are shown in Figure 2.
Figure 2
Survival analysis of anti-MDA5+ DM patients with ANA positivity. Survival analysis revealed no statistically significant differences in the mortality rates among the three patient groups with different anti-MDA5 antibody titers (log-rank p = 0.085)
We analyzed the clinical characteristics of 117 anti-MDA5+DM patients in the ANA-negative subgroup. Patients were divided into three groups based on different anti-MDA5 antibody titers, with 31 cases in the MDA5+ group, 21 cases in the MDA5++ group, and 65 cases in the MDA5+++ group. There were no significant differences in gender, age, course of the disease, and clinical features among the three groups of patients. In terms of LDH levels, compared to the MDA5+ group, levels were higher in the MDA5+++ group (p < 0.05). Regarding serum ferritin levels, compared to the MDA5++ group, levels were higher in the MDA5+++ group (p < 0.05). In terms of mortality rate, compared to the MDA5+ group, patients in the MDA5+++ group had a higher mortality rate and a worse prognosis (p < 0.05) (Table III).
Table III
Baseline characteristics of three groups of patients with different anti-MDA5 antibody titers among 117 ANA-negative patients
* Values statistically significant at p < 0.05. anti-MDA5+ DM – anti-melanoma differentiation-associated gene 5 positive dermatomyositis, RPILD – rapidly progressive interstitial lung disease, ALT – alanine aminotransferase, AST – aspartate aminotransferase, CK – creatine kinase, LDH – lactate dehydrogenase, ESR – erythrocyte sedimentation rate, CRP – C-reactive protein, ANA – antinuclear antibody, Anti-ARS – anti-aminoacyl-tRNA synthetase.
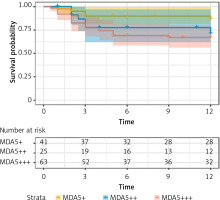
The follow-up period of 117 ANA-negative patients ranged from 0.5 months to 12 months, with a median follow-up time of 10 months. Among them, 29 patients died during the follow-up period (1 year). The survival times of three groups of patients (ANA+/MDA5+; ANA+/MDA5++; ANA+/MDA5+++) were compared. The results showed that there was a statistically significant difference in the mortality rate among the three groups with different MDA5 antibody titers (log-rank p = 0.025), especially between MDA5+++ and MDA5+ patients (p = 0.015). The survival rate of MDA5+++ patients was significantly lower than that of MDA5+ patients (log-rank p = 0.025). The Kaplan-Meier curves are shown in Figure 3.
Figure 3
Survival analysis of anti-MDA5+ DM patients with ANA negativity. Survival analysis showed a statistically significant difference in the mortality rate between MDA5+++ and MDA5+ patients (log-rank p = 0.025)
In 117 cases of anti-MDA5+ DM patients with negative ANA, univariate and multivariate Cox regression analyses were used to evaluate the risk factors for mortality. The results indicated that elevated levels of LDH and CRP, higher anti-MDA5 antibody titers, and the presence of RPILD were significantly associated with poor prognosis, with hazard ratios of 1.001 (95% CI: 1.001, 1.002, p = 0.009), 1.015 (95% CI: 1.003, 1.027, p = 0.015), 3.879 (95% CI: 1.160, 12.972, p = 0.028), and 11.757 (95% CI: 4.733, 29.205, p < 0.001), respectively. Subsequently, all of the meaningful variables that had been applied as covariates were included in the multivariate analysis. The multivariate regression analysis revealed that elevated LDH levels and the presence of RPILD were associated with poor prognosis, with hazard ratios of 1.002 (95% CI: 1.001, 1.003, p = 0.020) and 13.694 (95% CI: 15.032, 37.267, p < 0.001), respectively (Table IV).
Table IV
Predictors of survival in 117 ANA-negative anti-MDA5+ DM patients
Discussion
Our patients were enrolled from the Nanjing Medical University Myositis-Associated Interstitial Lung Disease cohort, which is a multicenter, retrospective, longitudinal cohort with data collected from ten tertiary hospitals in the East China region. In this large-scale cohort study, we investigated the clinical characteristics and prognosis of patients with anti-MDA5+ DM using representative and comprehensive data on ANA. Our study has four major findings: (i) ANA is highly prevalent (52.4%) in anti-MDA5+ DM patients. (ii) Among ANA-positive patients, the coexistence of anti-Ro52 antibodies and anti-ARS antibodies is significantly more common. (iii) Compared to the MDA5+ group, within the ANA-positive subgroup, there is a higher incidence of RPILD in MDA5+++ patients. In the ANA-negative subgroup, MDA5+++ patients have a higher mortality rate and worse prognosis. Elevated LDH levels and the presence of RPILD were associated with poor prognosis in ANA-negative anti-MDA5+ DM patients, with hazard ratios of 1.002 (95% CI: 1.001, 1.003, p = 0.020) and 13.694 (95% CI: 15.032, 37.267, p < 0.001), respectively. (iv) Our survival analysis results revealed no statistically significant difference in survival rates between ANA-positive and ANA-negative patients in the entire cohort of anti-MDA5+ DM patients (log-rank p = 0.890). Although ANA positivity may be associated with poor prognosis in other autoimmune diseases such as autoimmune hepatitis, primary biliary cholangitis, and thyroid dysfunction [18], our study suggests that ANA positivity is not a key factor influencing survival rates in this specific group of anti-MDA5+ DM patients.
Only a few studies have attempted to explore the impact of ANA positivity on DM [9], and due to small sample sizes, the research results are contradictory [19–21]. The effect of ANA positivity on MDA5+ DM remains unclear. ANA testing is typically used for suspected DM patients, with research showing 50%-78% of confirmed DM cases being ANA positive [7, 8]. In our study, the baseline ANA positivity rate among 246 anti-MDA5+ DM patients was 52.4%, similar to the 50–78% positivity rate found in previous series of studies [7, 8]. Hoesly et al. conducted a retrospective cohort study of 231 DM patients to explore differences in the incidence of myositis, risk of malignancy, and clinical characteristics [9]. Among the 231 patients, 140 (61%) tested positive for ANA. Compared to ANA-negative patients, ANA-positive patients had lower frequencies of dysphagia (15% vs. 26%; p = 0.033) and photosensitivity rash (38% vs. 53%; p = 0.026). In multivariable analysis, a close association was found between ANA positivity and lower likelihood of malignancy (odds ratio = 0.16; p < 0.001). Conversely, ANA positivity was not associated with myositis (amyopathic disease) (odds ratio = 0.94; p = 0.87) [9]. Li et al.’s study showed that compared to ANA-negative DM patients, ANA-positive DM patients were more likely to have elevated creatine kinase, abnormal immunoglobulins, and an accelerated erythrocyte sedimentation rate (p < 0.05) [21]. In our study, the ANA-positive group had significantly higher rates of anti-Ro52 and anti-ARS antibodies compared to the ANA-negative group, but ALT levels were significantly lower in the ANA-positive group. No significant differences were observed between the two groups in terms of overall survival, RPILD occurrence rate, age, disease duration, and clinical characteristics.
This study has several clinical implications. Firstly, subgroup analysis revealed a higher incidence of RPILD in MDA5+++ patients in the ANA-positive subgroup, while MDA5+++ patients had a higher mortality rate and poorer prognosis in the ANA-negative subgroup. Elevated LDH levels and the presence of RPILD were associated with poor prognosis in ANA-negative anti-MDA5+ DM patients. This emphasizes the importance of detailed analysis of subgroups based on different antibody positivity rates, aiding clinicians in better identifying high-risk patients. In particular those with high titers of anti-MDA5 antibody should be closely followed up for RPILD. Secondly, the results indicate differences in the distribution of different antibody positivity rates in anti-MDA5+ DM patients, especially with significantly higher positivity rates of anti-Ro52 antibodies and anti-ARS antibodies in the ANA-positive group compared to the ANA-negative group. This helps doctors to better assess the patient’s condition and prognosis during diagnosis and treatment, and the interactions and correlations between different antibodies warrant further in-depth study. Thirdly, the study found that in the ANA-positive group, ALT levels were significantly lower than in the ANA-negative group, which may reflect differences in liver function between the different antibody positivity rate groups. This indicates the need for close monitoring of liver function indicators in the management of anti-MDA5+ DM patients to promptly detect and address potential liver function abnormalities. In conclusion, based on the differences among different antibody positivity rate groups, doctors can better formulate treatment plans, including addressing liver function abnormalities, more proactive monitoring and treatment for high-risk patients, and better evaluation of patient prognosis.
This study has several limitations. Firstly, we were unable to obtain information on the titers of ANA and fluorescence patterns. However, our focus was on the association between ANA and anti-MDA5+ DM. Secondly, this study only involved patients from the Nanjing Medical University Myositis-Associated Interstitial Lung Disease cohort, so the results may not be generalizable, and more data from different regions and hospitals are needed to validate the reliability of the conclusions. Thirdly, this study was retrospective, and information on medication treatment was not collected, which may introduce recall bias and data incompleteness. Therefore, caution should be exercised in interpreting the results. However, we focused on and included clinical characteristics and relevant antibody information of anti-MDA5+ DM, as these factors are among the most important clinical data in anti-MDA5+ DM patients. Finally, residual confounders may still have an impact on this study, and further comprehensive research is needed to validate our results. The strength of this study lies in its relatively large study cohort and the use of multicenter data from the entire province, which is more representative of the general population compared to most hospital-based studies.
In conclusion, this relatively large population-based cohort study suggests that ANA is frequently found in patients with anti-MDA5+ DM, and that high titers of anti-MDA5 antibodies are associated with mortality and RPILD.