Portal venous system thrombosis (PVST) refers to the development of clots within the main portal vein, intrahepatic portal vein branches, mesenteric vein, and/or splenic vein. It is an uncommon condition often associated with hypercoagulable state and intra-abdominal infection [1]. Acute PVST often causes abdominal pain secondary to intestinal ischemia. When intestinal necrosis develops, abdominal pain becomes more severe with high mortality, and bowel resection is often required. Anticoagulation has been regarded as the first-line choice for treating PVST [2]. However, more than 60% of non-cirrhotic and non-malignant patients with PVST cannot achieve portal vein recanalization after adequate anticoagulation [3]. Additionally, the rate of recurrent thrombosis is up to 40% in patients receiving anticoagulation alone, regardless of the duration of anticoagulation [4]. In this setting, thrombolysis has been attempted for improving the outcomes of acute PVST. Herein, we present a case of acute PVST secondary to intra-abdominal infection which was effectively treated with systemic thrombolysis combined with anticoagulation and antibiotics.
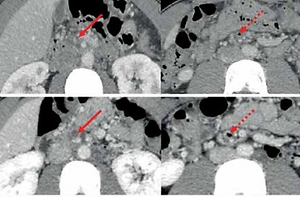
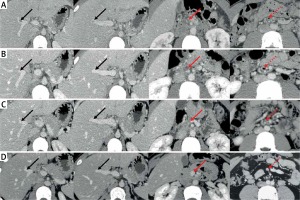
A 24-year-old solider with no significant medical history was admitted to the Department of Emergency of our hospital due to aggravating severe abdominal pain accompanied by fever, nausea, and vomiting for 4 days. He did not have any family history of venous thromboembolism. On day 1 of admission, laboratory tests demonstrated significantly elevated white blood cell counts, neutrophil counts, and C-reactive protein (Table I). Plain computed tomography (CT) scans also showed increased mesenteric density, suggesting a diagnosis of peritonitis. Intravenous injection of ertapenem at a dose of 1000 mg was given. On day 2 of admission, contrast-enhanced CT scans were performed after a consultation with gastroenterologists, and showed no contrast agent filling within the right portal vein (RPV) and superior mesenteric vein (SMV), but mesenteric venous gas (Figure 1 A). Thus, a diagnosis of acute PVST was made. On the same day, the subcutaneous injection of enoxaparin sodium was initiated at a dose of 40 mg twice per day and intravenous injection of levofloxacin was given at a daily dose of 500 mg. On day 5 of admission, he was transferred to the Department of Gastroenterology. Laboratory tests showed a reduction in white blood cell counts, neutrophil counts, and C-reactive protein (Table I). On day 8 of admission, contrast-enhanced CT scans showed that RPV thrombosis was recanalized, but SMV thrombosis remained occluded and mesenteric venous gas persisted (Figure 1 B). Thus, systemic thrombolysis was recommended. After this patient and his relatives’ informed consent forms were obtained, the intravenous injection of urokinase at a dose of 300,000 IU was added to enoxaparin sodium at a dose of 40 mg twice per day, and levofloxacin injection was changed to ceftriaxone sodium at a dose of 1000 mg twice per day. On day 11 of admission, this patient’s abdominal pain improved significantly. On day 15 of admission, urokinase was discontinued, but enoxaparin sodium was continued. During systemic thrombolysis, the patient did not experience any bleeding episode. On day 31 of admission, his abdominal pain completely disappeared. Enoxaparin sodium was replaced with oral rivaroxaban 10 mg twice per day. Contrast-enhanced CT scans showed that SMV thrombosis was slightly improved, and mesenteric venous gas disappeared (Figure 1 C). Then, he was discharged. After 3-month anticoagulation with rivaroxaban, contrast-enhanced CT scans showed that SMV became patent (Figure 1 D). Laboratory tests showed the normalization of white blood cell counts, neutrophil counts, and C-reactive protein (Table I).
Table I
Laboratory tests in this patient
Figure 1
Axial computed tomography images in this patient. A – On day 2 of admission, CT images demonstrated occlusive thrombosis within the right portal vein (RPV) (solid black arrow) and superior mesenteric vein (SMV) (solid red arrow) and mesenteric venous gas (dotted red arrow). B – On day 8 of admission, CT images demonstrated partially recanalized RPV (solid black arrow), but SMV (solid red arrow) thrombosis remained occluded and mesenteric venous gas persisted (dotted red arrow). C – On day 31 of admission, CT images demonstrated that SMV (solid red arrow) thrombosis was slightly improved, and the mesenteric venous gas disappeared (dotted red arrow). D – After 3-month anticoagulation with rivaroxaban, CT images demonstrated completely recanalized SMV (solid red arrow) without mesenteric venous gas (dotted red arrow)
Acute abdomen caused by PVST has been increasingly recognized. However, systemic thrombolysis has been rarely attempted for the treatment of acute PVST in clinical practice due to its potential bleeding risk [2]. Our case was diagnosed with acute symptomatic PVST according to the time of onset, and still presented with abdominal pain and occlusive mesenteric vein thrombosis after 1-week anticoagulation alone. Finally, he underwent systemic thrombolysis, which may increase the possibility of thrombus recanalization.
PVST can be caused by various local and/or systemic thrombophilic factors, including pancreatitis, portal vein injury during surgery, and intra-abdominal infection [5]. In our case, pylephlebitis due to intra-abdominal infection should be a major predisposing factor for PVST because he developed persistent fever two days before the occurrence of abdominal pain, and had higher levels of white blood cell count, neutrophils, and C-reactive protein at admission. Intra-abdominal infection can cause inflammation within and around splanchnic vessels via the procoagulant effects of gut anaerobes and neutrophil extracellular trap formation in local venules, thereby developing PVST [6]. Notably, our case developed mesenteric venous gas, which might be attributed to gas-producing microorganisms within the hepatic vascular or infection focus that enters into the splanchnic circulation [7]. Additionally, screening for other thrombotic risk factors revealed a heterozygous mutation in methionine synthase reductase A66G polymorphism and a homozygous mutation in plasminogen activator inhibitor 1 4G/5G in our case, which should also be concomitant risk factors for PVST [8, 9].
Antibiotics are the mainstay treatment for pylephlebitis. Anticoagulation has also been shown to further improve portal vein recanalization [10, 11]. If there is no response to anticoagulation therapy in patients with recent and extensive PVST and signs of intestinal ischemia or infarction, thrombolytic therapy may be effective [12]. However, the dosage and duration of thrombolysis have not been clearly recommended by the guidelines. In a prospective cohort study [13], 9 cirrhotic patients with recent PVST received continuously intravenous infusion of recombinant tissue-type plasminogen activator (rtPA) at a dose of 0.25 mg/kg/day combined with subcutaneous injection of low molecular weight heparin for 4–7 days. The rate of PVST recanalization was 88.9%. In a retrospective cohort study [14], 33 patients with acute PVST were treated with intravenous injection of 750,000 IU/day streptokinase or 100–150 mg/6–12 h rtPA for 2–3 days followed with heparin infusion, and then received oral anticoagulant for 12 months after discharge. The rate of PVST recanalization was 69.7%. Until now, urokinase has never been employed for systemic thrombolytic therapy in patients with acute PVST. Considering that PVST was more severe in our case, urokinase was given at a greater dosage for a longer duration as compared to its recommended dosage and duration for deep vein thrombosis [15]. Notably, his abdominal pain disappeared completely without any bleeding at discharge.
There are several limitations in this case report. First, considering that this patient was thin with a weight of 50 kg and the specification of enoxaparin is 40 mg per dose, a dosage of 40 mg once was finally selected, which may be associated with suboptimal outcomes of anticoagulant therapy alone [16–18]. Second, we could not know if this patient has a JAK2 V617F mutation, because the gene mutation detection is not available at our hospital. Third, we have not re-examined the lupus anticoagulant in this patient yet. Thus, a diagnosis of antiphospholipid syndrome could not be made.
To the best of our knowledge, our case may be the first report of systemic thrombolysis combined with anticoagulation and antibiotics for acute PVST with pylephlebitis. Generally, systemic thrombolysis provides a non-invasive approach with its benefits in alleviating symptoms and achieving portal vein recanalization more rapidly [19]. But it should be cautiously employed in young patients with acute PVST secondary to intra-abdominal infection who are free of bleeding risk factors and have no response to anticoagulation and antibiotics alone. Additionally, transjugular intrahepatic portosystemic shunt should be selectively considered in such patients [20].