Introduction
Estrogens exert a pleiotropic effect on a woman’s whole body: the skin, adipose tissue, bones, the central nervous system, cardiovascular and digestive systems, not only on the reproductive system [1, 2]. Previous reports identified the role of estrogens in the regulation of synaptogenesis in the region of the dorsal hippocampus in female rats [3]. The naturally occurring low levels of estrogens noted before and after menopause may be related to cognitive impairments or dementia [4]. Hence, hormone replacement therapy (HRT) can reduce the risk of dementia in postmenopausal women, as confirmed by the meta-analysis of LeBlanc et al. [5].
Estrogen interacts with two estrogen receptors: estrogen receptor-α (ERα) and estrogen receptor-β (ERβ), encoded by ESR1 and ESR2 genes, respectively. Two polymorphisms within ESR1 (rs9340799 – 351A>G (XbaI) and rs2234693 – 397T>C (PvuII), separated by only 46 base pairs) are noted to play a role in several diseases [6–8]. It is also suggested that both ESR1 polymorphisms may have an impact on cognitive functioning [9, 10]. However, there are also contrary results in the topic [11].
Available data establish a clear relationship between estrogens and apolipoprotein E (APOE). Earlier results also showed that the APOE ε4 allele was related to a range of disorders, including lipid profile [12], Alzheimer disease (AD) [13, 14], and coronary artery disease, often in the presence of other candidate polymorphisms [15]. It was also observed that healthy individuals carrying the APOE ε4 allele had significantly worse overall verbal episodic memory than healthy non-carriers [16]. A study on mouse cortical neurons showed that estradiol influenced neurite growth through an ApoE-dependent mechanism; therefore, HRT may have a different impact on chronic neurological diseases due to the presence of a particular APOE genotype [17].
The aim of the present study was to analyze the impact of interactions between APOE and ESR1 polymorphisms on cognitive functioning in postmenopausal women.
Material and methods
Study group
The study was conducted in 2014 at the Institute of Rural Health in Lublin, Poland. The study group comprised 266 women from south-eastern Poland. The inclusion criteria were: age 50–65 years, with a minimum period of 2 years since the last menstrual period. The exclusion criteria were: any chronic diseases within the last 5 years, medical history of mental diseases, addiction to drugs and/or alcohol, diagnosed disease entity with the symptoms of dementia, current or past use of HRT, severe menopausal symptoms according to the Kupperman menopausal index [18]. A brief Montreal Cognitive Assessment (MoCA) test was performed in order to exclude women who presented with features of dementia. Only women who obtained scores of at least 26 were included in the study.
Computerized neurocognitive assessment software for CNS-Vital Signs
Assessment of cognitive functions [19] was performed based on the diagnostic equipment, CNS Vital Signs (1829 East Franklin Street, Bldg 500, Chapel Hill NC 27514, 919-933-0932). The instrument, consisting of a battery of computer tests, is standardized, and was subjected to a full validation procedure. It has many cultural and language adaptations, including one for Polish. Nine cognitive functions – Memory, Verbal Memory, Visual Memory, Processing Speed, Executive Functioning, Psychomotor Speed, Reaction Time, Complex Attention, Cognitive Flexibility – were assessed. The CNS VS test provides the Neurocognitive Index based on five cognitive functions: memory, psychomotor speed, reaction time, attention, and cognitive flexibility. Standard scores of the NCI and of nine cognitive functions were analyzed and interpreted as: above average (> 109), average (90–109), low average (80–89), low (70–79), very low (< 70), with higher values of standard scores indicating better cognitive functions.
DNA isolation
Genomic DNA isolation was derived from 0.2 ml of human blood by the QIAamp DNA Blood Mini Kit (Qiagen, USA), as per the producer’s instructions. The amount and purity of the extracted DNA were measured using the NanoDrop spectrophotometer.
APOE polymorphism
Multiplex polymerase chain reaction (PCR) was carried out according to Yang et al. [20], with some modifications. PCR reactions were made in a single reaction tube with six primers, including two common primers and two specific primers for each of two single nucleotide polymorphism (SNP) sites. The multiplex PCR reaction was carried out in a 50 μl reaction volume containing the following mix of reagents: 1.25 U Taq DNA polymerase, 1× PCR buffer containing 15 mM MgCl2 and 1× Q buffer (all from Qiagen, USA), 0.2 mM each of dNTP (Fermentas, Lithuania), 0.5 μM of each of six primers: FO, RO, FI-1, RI-1, FI-2, RI-2 (Eurogentec, Seraing, Belgium), nuclease-free water (Applied Biosystems, USA) and 5 μl of DNA. The reaction was performed in a C1000 Thermal Cycler (Bio-Rad) under the following conditions: initial denaturation at 95°C for 5 min, then 35 cycles (denaturation 95°C for 30 s, annealing at 60°C for 30 s, elongation at 72°C for 60 s); the final extension step is at 72°C for 7 min. The reaction products were detected in 2.5% agarose gels in the standard electrophoresis conditions. After ethidium bromide staining, the strips were read under UV light. The size of the amplified DNA fragment, using two common outer primers (FO and RO), was 514 bp. Obtained DNA amplicons flanked by each of two sets of allele-specific inner primers (FI-1/RI-1 and FI-2/RI-2) showed different types of polymorphisms: 444 bp, 307 bp and 115 bp for ε3/ε4; 307 bp and 115 bp for ε3/ε3; 444 bp and 307 bp for ε4/ε4; 307 bp, 253 bp and 115 bp for ε2/ε3; 444 bp, 307 bp, 253 bp and 115 bp for ε2/ε4.
ESR1 polymorphisms
Polymorphisms of ESR1 were determined using the restriction fragment length polymorphism (RFLP-PCR) method. PCR reaction was performed in a total amount of 50 μl containing: 1 U (1 μl) of DNA polymerase (Biotools), 1 PCR buffer (5 μl) containing 15 mM MgCl2 (Biotools), 2.5 μl 2 mM dNTPs (final concentration 0.1 mM) (Fermentas, Vilnius, Lithuania), 1 μl of 10 μM of each of the two primers, 34.5 μl nuclease-free water (Applied Biosystems Inc., USA) and 5 μl of genomic DNA. The reactions were performed in a C1000 Thermal Cycler (Bio-Rad) and consisted of the initial denaturation (3 min at 95°C) and 30 cycles, each of which included the proper denaturation (30 s at 95°C), primer annealing (50 s at 62°C), elongation (50 s at 72°C), and the final elongation (7 min at 72°C). Electrophoresis was performed in 2% agarose gel in standard conditions. The products of PCR (1372 bp) were digested overnight at 37°C using 2 separate restriction enzymes for determining the polymorphisms: PvuII (c.454-397 T>C) and XbaI (c.454-351 A>G). The products of restriction were electrophoresed in 2.5% agarose gel.
The alleles of the XbaI polymorphism were defined as A and G: heterozygote AG (fragments: 1372 bp, 936 bp, and 436 bp), homozygote GG (fragment: 1372 bp), and homozygote AA (fragments: 936 bp and 436 bp). The alleles of the PvuII polymorphism were defined as T and C: heterozygote TC (fragments: 1372 bp, 982 bp, and 390 bp), homozygote TT (982 bp and 930 bp), and homozygote CC (1372 bp).
Statistical analysis
The data were statistically analyzed using Statistica software. We estimated mean values (M) with standard deviations (SD) for continuous variables, and absolute (n) and relative numbers (%) of occurrence of items for categorical variables. Two-way analysis of variance was used to compare cognitive functions versus APOE and ESR1 polymorphisms. F statistics were used to test three different hypotheses: APOE polymorphism affects cognitive functions; ESR1 polymorphism affects cognitive functions; and the interaction between APOE and ESR1 polymorphisms affects cognitive functions. Due to the small sample sizes of women with ε4/ε4 and women with ε3/ε4, they were combined together for statistical analysis. One-way analysis of variance was used to compare cognitive functions versus interaction between XbaI and PvuII of ESR1 polymorphisms due to the very small sample sizes or even empty cells in cross-sections.
The value of p ≤ 0.05 was considered to indicate a significant difference.
Informed consent for participation in the study was obtained from all women. The study was approved by the Ethics Committee of the Institute of Rural Medicine in Lublin, Poland.
Results
Study group characteristics
A total of 266 postmenopausal women, aged 50-65 years, with an average age of 56.6 ±3.4 years, of mostly secondary education, were examined in the study. The majority of them were carriers of ε3/ε3 APOE polymorphism, with approximately half of them possessing AG genotype of the ESR1 XbaI polymorphism and a little fewer having TC genotype of the ESR1 PvuII polymorphism (Table I).
Table I
Study group characteristics
Cognitive functions vs. APOE polymorphism
NCI and five of nine cognitive functions – executive functioning, psychomotor speed, reaction time, complex attention and cognitive flexibility – depended on APOE gene polymorphism (Table II). NCI was assessed as average in women with ε3/ε3 genotype, as low average in women with ε3/ε3, as low in women with ε3/ε4, and very low in women with ε4/ε4. Executive functioning and cognitive flexibility were assessed as average in women with ε3/ε3 genotype, as low in women with ε3/ε3 and ε3/ε4, and very low in women with ε4/ε4. Psychomotor speed was assessed as average in women with ε3/ε3 genotype, as low in women with ε3/ε3, and very low in women with ε3/ε4 and ε4/ε4. Reaction time was assessed as average in women with ε3/ε3 genotype, and as low average in women with ε3/ε3, ε3/ε4 and ε4/ε4. Complex attention was assessed as average in women with ε3/ε3 genotype, as low in women with ε3/ε3, and very low in women with ε3/ε4 and ε4/ε4.
Table II
Cognitive functions vs. APOE polymorphism
Cognitive functions vs. ESR1 polymorphisms
Three cognitive functions – processing speed, executive functioning and cognitive flexibility – depended on ESR1 PvuII polymorphism (Table III). Processing speed was better in women who were TT homozygotes than in women with TC and CC genotypes. Executive functioning and cognitive flexibility were lower in women with TT genotype than in those with TC and CC genotypes.
Four cognitive functions – memory, verbal memory, visual memory and processing speed – depended on ESR1 XbaI (Table IV). Women with AA and AG genotypes had better above-mentioned cognitive functions than those who were GG homozygotes.
Table III
Cognitive functions vs. ESR1 PvuII polymorphism and vs. interaction with APOE polymorphism
Table IV
Cognitive functions vs. ESR1 XbaI polymorphism and vs. interaction with APOE polymorphism
Cognitive functions vs interaction between APOE and ESR1 polymorphisms
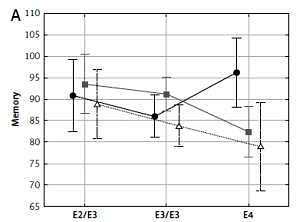
Memory, visual memory, processing and psychomotor speeds depended significantly on the interaction between APOE and ESR1 PvuII polymorphisms (Table III and Figure 1). In women carrying the C allele of the PvuII polymorphism (TC and CC) cognitive functions were the best in the presence of the ε2/ε3 genotype, lower if they possessed ε3/ε3 and the lowest if they possessed the ε4 allele. Different relations were observed in women with TT genotype of the PvuII polymorphism – cognitive functions were not decreased in women with the ε4 allele.
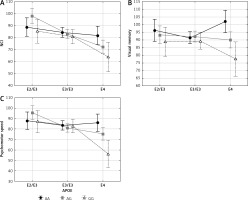
Figure 1
Significant effect of interaction between APOE and ESR1 PvuII polymorphisms on cognitive functions
Similarly, interaction between APOE and ESR1 XbaI polymorphisms had a significant impact on NCI, visual memory and psychomotor speed (Table IV and Figure 2). In women with AG and GG genotypes of the XbaI polymorphism cognitive functions were the best if women possessed APOE ε2/ε3 genotype, lower in the presence of ε3/ε3 genotype, and the lowest if they possessed the ε4 allele. Different relations were observed in women having AA genotype of the XbaI polymorphism for which cognitive functions were not decreased in women with the ε4 allele.
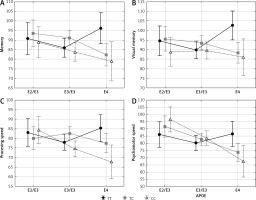
Cognitive functions vs interaction between PvuII and XbaI polymorphisms of ESR1 gene
NCI and six cognitive functions differed between genotypes of PvuII and XbaI combined (Table V). Women who simultaneously carried CC PvuII and GG XbaI genotypes had the lowest cognitive functions mentioned above, women with TC and AA or CC and AG demonstrated the best, while women with TT and AA or TC and AG had moderate outcomes.
Table V
Cognitive functions vs. interactions of PvuII and XbaI of ESR1 polymorphism
Discussion
Old age is a risk factor for cognitive decline. In the aging population, there is a high prevalence of mild cognitive impairment (MCI), which increases the risk of AD. It is forecast that by 2050 over 100 million people will develop such cognitive problems. MCI and dementia have consequences, especially for affected people but also for their caregivers, the health care delivery system and society in general. The problem especially applies to women after menopause when levels of estrogen, which have a neuroprotective effect, begin to drop significantly. In about 80% of women in the postmenopausal state neuronal degeneration may lead to difficulties in concentrating, overreacting or forgetfulness [21]. Menopause affects various activities of women’s life [22]; among its effects, a decline in neurocognitive functions during the peri- and postmenopausal periods is observed [23].
The latest experimental data indicate some morphological changes in the hippocampus of postmenopausal female mice, i.e. mitochondrial damage, lipofuscin deposition and microtubule degradation [24]. In turn, in the study of Albert et al. [25], the effect of estrogen on the hippocampus and cognitive function was confirmed. The authors observed increased bilateral posterior hippocampal voxel-based gray-matter volume in women taking 2 mg of estrogen, while in women who received a placebo or 1 mg of estrogen no such effect was noted [25].
Age-related cognitive functions depend on various genetic risk factors, including APOE and ESR1. The first purpose of the present study was to analyze whether there are relations between both APOE and ESR1 polymorphisms and several cognitive functions in a large group of postmenopausal women. We observed that women with ε4/ε4 show significantly lower executive functioning, psychomotor speed, reaction time, complex attention as well as cognitive flexibility compared to those with other APOE genotypes. The study of Schoemaker et al. [26] revealed significant positive correlations between familiarity performance and the volume of the perirhinal and entorhinal cortices as well as between recollection performance and hippocampal volume in carriers of APOE ε4. Levels of ApoE were found to be higher in women and related to lifespan and cognitive function [27]. In turn, in elderly women who were carriers of the APOE ε4 allele and exposed to traffic pollution, cognitive impairment in the visuospatial domain was demonstrated [28]. Similarly, the presence of some depressive symptoms has a significant effect on cognitive impairment, which is increased in APOE ε4 carriers [29]. It was also demonstrated that in post-menopausal women the correlation between CRP level and cognitive functions may be modified by apolipoprotein E genotypes [30]. In addition, an enhanced negative effect of testosterone on cognition was found in postmenopausal women with at least one APOE ε4 allele [31].
In the case of ESR1 polymorphisms we observed that memory, verbal memory, visual memory and processing speed were at the lowest level in G allele homozygotes of the XbaI polymorphism. In contrast, women with CC genotype of the PvuII polymorphism had lower processing speed and cognitive flexibility but higher executive functioning. Data regarding the ESR1 polymorphisms are often contradictory. Some studies show such a relationship while others do not. In the study of Elcoroaristizabal et al. [32] an association between the APOE ε4 allele and amnesic mild cognitive impairment was found while no relation to cognition was observed for ESR1 polymorphisms in elderly women. In contrast, in the same study the polymorphisms within the ESR2 gene were associated with lower cognitive performance [30]. Similar findings were described by Ryan et al. [33]. No relationship between ESR1 polymorphisms and AD or vascular dementia was found in older Jewish women [34]. On the other hand, the study of Ma et al. [9] analyzing the same relationship between twenty ESR1 polymorphisms and cognition in a group of Chinese older adults demonstrated that eight polymorphisms may be considered as markers for episodic memory decline at an earlier stage. In the study of Yaffe et al. [35] cognitive impairments were more likely to occur in women than men. The rs9340799 (XbaI) polymorphism in the ESR1 gene was associated with cognitive decline in univariate analysis and after adjustment for age, educational level and score of 3MS (Modified Mini-Mental State) the correlation was slightly stronger. Such a relationship was not observed in the case of men [33].
The main goal of our study was to analyze the impact of the interaction between APOE and ESR1 polymorphisms on cognitive functions in the analyzed women. In the case of interactions between ESR1 PvuII polymorphism and APOE, significant differences in the domains of memory, visual memory, processing and psychomotor speeds were observed. The lowest cognitive functions characterized women with PvuII TC and/or CC genotypes with simultaneous presence of at least one APOE ε4 allele. The best cognition was observed for women with TC and/or CC and ε2/ε3 genotypes. Otherwise, women with PvuII TT seemed to be protected from cognitive decline even in the presence of the APOE ε4 allele, and their functions did not get lower with the APOE genotypes. Similar findings in regard to the interactions between ESR1 XbaI and APOE polymorphisms were demonstrated. Women with XbaI AG and/or GG genotypes and the APOE ε4 allele had the lowest cognition compared to women with XbaI AG and/or GG genotypes but having ε2 or ε3 alleles. In women with XbaI AA and APOE ε4, smaller deterioration in NCI as well as better visual memory and psychomotor speed was observed.
The study of Fernández-Martínez et al. [13] showed increased risk of mild dementia and AD in the simultaneous presence of the APOE ε4 allele and polymorphic variants of both XbaI and PvuII polymorphisms within the ESR1 gene. Opposite to our study, Fehsel et al. [11] found no relation of ESR1 polymorphisms and cognition, but minor variants of ESR2 polymorphisms had a significant impact on executive function in women carrying the APOE ε4 allele. In addition, the authors observed that air pollution increased the risk of cognitive decline in women. In male patients with AD, ESR1 PP and XX genotypes increased the risk of the disease and the risk was especially high in those with at least one APOE ε4 allele (OR = 13.3) [36]. It was also demonstrated that wild-type genotypes (i.e. PP and XX) of ESR1 of PvuII and XbaI polymorphisms were associated with a faster cognitive decline in women with AD. Also, these genotypes decreased ApoE levels in male patients [36].
Cognitive functions in postmenopausal women may also correlate with biochemical, environmental or social factors. Previously, the impact of some of them, i.e. education, health behaviors, serum hormones and CRP protein concentrations, on cognitive functions was analyzed [23, 30, 31, 37]. The impact of cardiovascular risk factors on cognition was also considered [38, 39]. However, further studies are needed to understand the wide range of risk factors of cognition impairment as well as the interactions between them.
In conclusion, the AA and TT genotypes of estrogen receptor α polymorphisms protect postmenopausal women having the ε4 allele of the APOE polymorphism from low cognitive functions. NCI, memory, verbal memory, processing speed, psychomotor speed as well as reaction time were decreased the most in postmenopausal women who simultaneously carried the CC PvuII and GG XbaI genotypes.