Introduction
Children experience fever more frequently than adults. Fever is a crucial immune response of the body that helps us eliminate invading pathogens [1], and is a common symptom in various pediatric diseases, particularly infectious diseases [2]. In general, when febrile children present to a clinic, physicians focus on determining and treating the underlying cause of the pyrexia, whereas parents are more concerned about alleviating the fever itself. Fever is also a common reason for visiting physicians. This phenomenon is called “fever phobia” [3]. The role of antipyretic medication is to ease the child’s discomfort caused by fever and prevent dehydration [4]. The most commonly recommended antipyretic drugs are acetaminophen and ibuprofen [5]. Acetaminophen has a longer history, starting in the 1950s when it replaced aspirin to prevent Reye’s syndrome; currently, its labeled dose is 10–15 mg/kg every 4 h in children aged > 3 months [6]. Ibuprofen, approved for use in febrile children in 1989, is a common over-the-counter medication [7]; currently, its labeled dose is 5–10 mg/kg every 6–8 h in children aged > 6 months.
Ibuprofen is conventionally considered more effective than acetaminophen in the treatment of fever [8], because it has a longer duration of action than does acetaminophen (6–8 vs. 4 h). Nevertheless, acetaminophen demonstrates a low adverse effect risk [9, 10]. A series of studies have assessed acetaminophen and ibuprofen prescription in the pediatric population. However, to our knowledge, only two systematic reviews have discussed monotherapy of the two medications for febrile children, and both syntheses mixed febrile and non-febrile children [8, 11]. The earlier synthesis, in 2004, only analyzed 10 of the 17 included randomized controlled trials (RCTs) for febrile children, while the other RCTs did not focus on febrile children [8]. Since the meta-analysis also included RCTs regarding pain management in children, the safety of the two medications in the synthesis mixed febrile and non-febrile children. The other meta-analysis published in 2020 had a similar situation, and focused on children under 2 years old [11]. Moreover, six related RCTs on this topic had been published before the previous systematic reviews [12–17]. The most effective clinical practice for using these two antipyretic medications remains unclear [18].
To improve the confidence and understanding in using the two antipyretic medications, updated evidence including all relevant RCTs is required. The study aimed to assess the efficacy and safety of acetaminophen and ibuprofen in febrile children, and the elements of our research question in the PICO form are as follows:
Material and methods
To appropriately answer our research question, we performed the study adhering to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions [19]. We then referred to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses to report our study, including eligibility criteria, data source, evidence selection, data extraction, quality evaluation, data pooling, and result reporting [20]. The protocol for this study has been published on PROSPERO (CRD42020150731).
Eligibility criteria
Based on the PICO of this study, the authors defined primary eligibility criteria for evidence selection before the search. The inclusion criteria were as follows: (a) patients with fever, (b) population sample represented by a pediatric group aged < 18 years, (c) therapy comprised at least one arm of acetaminophen and the other arm of ibuprofen for antipyretic treatment, and (d) the study had to be an RCT since RCT is a better study design for evidence regarding effects of intervention according to the recommendation of Cochrane handbook [19]. We did not exclude evidence according to sex, disease, dosage, and administration route.
Data source and evidence selection
Potential studies were mainly identified from the Cochrane Database of Systematic Reviews (including the Cochrane Central Register of Controlled Trials (CENTRAL)), Embase, and PubMed databases by using relevant keywords about four core elements using the terms fever, pediatric, acetaminophen, and ibuprofen. The relevant keywords consisted of free text and medical subject headings. The Boolean operator “OR” was used to combine synonyms of each core element, and the operator “AND” was applied to connect the four search parts. References were screened using the primary search strategy without any filters for restricting study design, publication date, language, or age (Appendix 1). In addition, Google Scholar was also searched; however, it had no advanced search function. Reference lists of relevant systematic reviews and RCTs were also screened for potential eligible evidence. A final search was performed for potential evidence before March 2021.
To identify eligible evidence, two authors (N.G. and N.Y.S.) independently screened titles and abstracts to eliminate irrelevant references. Subsequently, the full text of the remaining references was retrieved and carefully reviewed. The two authors excluded references according to the eligibility criteria mentioned earlier, and they further eliminated gray literature without details about study design, medication information, baseline characteristics, or outcomes during the full-text review step. After the two-step screening process, the two authors simultaneously checked the eligible evidence for the present study. If there was any disagreement related to selected evidence, an experienced researcher was consulted, who also made all final decisions about the included evidence.
Data extraction and quality evaluation
All eligible evidence for this study was further reviewed for data extraction and quality assessment. The two authors individually extracted studies by publication year, information of trial designs, characteristics of samples, diagnostic measures of fever, the definition of fever, types of intervention, the termination point of the study, and outcome measures. Types of intervention were different dosages and administration routes of acetaminophen and ibuprofen. The primary outcome measures were nonfebrile count; mean body temperature at baseline and 1, 2, 4, and 6 h after administration of antipyretics based on the pharmacokinetics research [21, 22]; and adverse effects classified by the system (gastrointestinal, respiratory, neurologic, dermatologic, hematologic, ear, nose and throat, and hypothermia). They double-checked data mutually before analysis. Then, the two authors and an experienced researcher had a meeting to resolve disagreements between them through triple-checking and discussion.
Quality evaluation was based on data extraction. To assess the risk of bias, the Cochrane risk-of-bias tool 2 (RoB 2), first released in the Cochrane Handbook for Systematic Reviews of Interventions in 2016 and updated in 2019, was used [23, 24]. Because the RoB 2 adopts an outcome-oriented approach, the two authors evaluated bias from the randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and selection of the reported result in each trial of every outcome.
Data synthesis and analysis
Trial characteristics and patient demographic information were synthesized qualitatively, and relevant outcomes were combined quantitatively. Head-to-head meta-analysis in a random-effects model was performed for quantitative analysis. Because fever resolution and adverse event rates were dichotomous variables, their events and non-events with each medication were used for obtaining the risk ratios and 95% confidence intervals (CIs). The Peto odds ratio (POR) was also estimated when any sparse cell existed in the fever resolution or adverse event rates. Interpretation of pooled estimates using sparse data was mainly based on POR for statistical robustness [19]. Weighted mean differences (WMDs) between acetaminophen monotherapy and ibuprofen monotherapy were estimated based on means, standard deviations, and sample sizes of each variable for each medicine.
To evaluate the quality of the pooled data, small study effects and heterogeneity were tested. The funnel plot and Egger’s test were performed to detect small study effects within pooled fever resolution rates, the difference in body temperatures, and adverse event rates. Because body temperatures were reported for multiple time points, a treatment–time interaction model was assessed [25]. Heterogeneity among the included trials was detected using I 2 statistics. If I 2 ≥ 50% or p-value < 0.10 for any outcome, the pooled result was considered highly heterogeneous. Subgroups of measuring time and age range were further analyzed for statistical and clinical heterogeneity. All analyses were performed using STATA for Microsoft Windows (version 14).
After quantitative synthesis of fever resolution, body temperature, and adverse event rates, the Grading of Recommendation, Assessment, Development, and Evaluations (GRADE) was further applied to the overall judgment of each finding for clinical practice [26]. Results of the GRADE evaluation mainly consist of certainty of the evidence, relative effects, and comments. There are four levels of certainty of the evidence: Very Low (⊕◯◯◯), Low (⊕⊕◯◯), Moderate (⊕⊕⊕◯), and High (⊕⊕⊕⊕).
Results
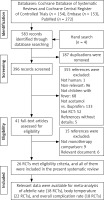
Our search yielded a total of 559 articles from the Cochrane (k = 154), Embase (k = 144), and PubMed (k = 261) databases. Four more articles were identified from reference lists of relevant systematic reviews and Google Scholar. Of these, 529 were excluded because they were duplicated (k = 183), were irrelevant (k = 88), did not include children with fever (k = 67), did not compare acetaminophen and ibuprofen monotherapies (k = 131), were not RCTs (k = 51), were abstracts without details (k = 5), and were other documents (k = 4). Of the remaining 34 articles, we further excluded 9 because they did not compare acetaminophen and ibuprofen monotherapies. Finally, 26 RCTs were included in this systematic review and meta-analysis [7, 12–17, 27–45]. The flow of article selection is illustrated in Figure 1.
Figure 1
Flow of selection of randomized controlled trials comparing acetaminophen and ibuprofen monotherapies
RCT – randomized controlled trial.
Characteristics and quality of included studies
The 26 included RCTs recruited 4137 children with fever from Africa, the Americas, Asia, and Europe. These trials were published between 1989 and 2020. Based on available data, the mean age ranged from 1.5 to 6.23 years. Most trials reported that baseline body temperature was > 38.5°C, except in a trial by Wilson with a temperature of approximately 37.5°C in each group and three trials without baseline body temperature [15, 36, 37, 44]. Eight trials clearly declared that children receiving antibiotics were excluded [13, 29–31, 34, 38, 40, 41]. Table I lists demographic information, and Appendix 2 presents the quality of the included RCTs according to the outcome of interest.
Table I
Characteristics of the included randomized controlled trials
Fever resolution rate and body temperature
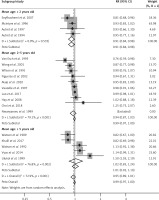
Data on fever resolution rates could be derived from 18 RCTs (n = 2734). The pooled estimate of fever resolution rates demonstrated that both acetaminophen (931/1329) and ibuprofen (1042/1405) monotherapies led to similar fever resolution rates (Figure 2), and the POR of fever resolution was 0.99 (95% CI: 0.97 to 1.001). However, the pooled estimate was highly heterogeneous (I 2 = 72.9%), and high heterogeneity existed between subgroups (I 2 = 52.6%, p < 0.10). Egger’s test did not detect small study effects (coefficient = −0.27; p > 0.05; Figure 3). In the subgroup of trials with a mean age of < 2 years, notably, acetaminophen monotherapy demonstrated a significantly lower fever resolution rate than did ibuprofen monotherapy (POR = 0.91, 95% CI: 0.84 to 0.98; I 2 = 0%). No significant difference in fever resolution rates between the two medications could be observed in the subgroups of trials with a mean age between 2 to 5 years old and above 5 years old.
Figure 3
Small study tests using funnel plot for fever resolution rate (A), Egger’s test for fever resolution rate (B), funnel plot for overall adverse event rate (C), and Egger’s test for overall adverse event rate (D)
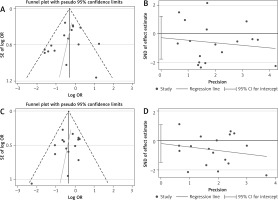
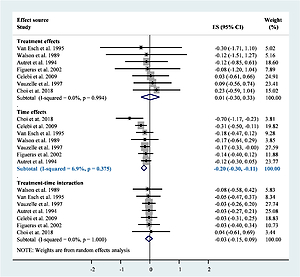
In total, 21 RCTs (n = 3569) reported body temperature data. The data for children’s body temperature at baseline (22 RCTs) and 1 (7 RCTs, n = 1069), 2 (7 RCTs, n = 1053), 4 (6 RCTs, n = 783), and 6 (5 RCTs, n = 606) h after medication administration were available (Appendix 3). Pooled estimates demonstrated no significant difference in body temperature between acetaminophen and ibuprofen at baseline and 1 and 2 h after intervention, whereas the pooled estimates were highly heterogeneous. Furthermore, compared with ibuprofen, acetaminophen demonstrated higher body temperatures 4 (WMD = 0.27; 95% CI: 0.08 to 0.45) and 6 (WMD = 0.23; 95% CI: 0.02 to 0.43) hours after administration. The treatment–time interaction model, however, demonstrated that the fever resolution effect was mainly from the time factor based on the available data (effect size = –0.20; 95% CI: –0.30 to –0.11; I2 = 6.9%). No significant effect from acetaminophen monotherapy or treatment–time interaction could be observed (Appendix 4).
Safety
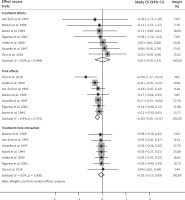
Data on adverse event rates were derived from 18 RCTs (n = 3286). A pooled estimate demonstrated that neither acetaminophen nor ibuprofen monotherapy demonstrated a significant difference in overall adverse event rates (POR = 0.97; 95% CI: 0.93 to 1.01; Figure 4), although high heterogeneity was observed in the pooled estimate (I2 = 67.1%) and between subgroups (I2 = 78.4%, p < 0.05). The pooled estimate of overall adverse event rates might not be considerably biased by small study effects (coefficient = −0.20; p > 0.05). Subgroup analysis demonstrated no significant differences in overall adverse event rates between acetaminophen and ibuprofen monotherapies in trials with a mean age of < 2 years old as well as mean age between 2 and 5 years old. Significant findings were observed only in trials with a mean age of > 5 years, where acetaminophen monotherapy demonstrated lower overall adverse event rates than ibuprofen monotherapy (POR = 0.71; 95% CI: 0.58 to 0.87; I 2 = 0%). Table II shows the summary of findings and certainty of evidence regarding the effects of acetaminophen and ibuprofen on afebrile rate and overall complication.
Table II
Summary of findings
| No. of participants (studies) | Certainty of theevidence (GRADE) | Relative effects(95% CI) | Comments |
|---|---|---|---|
| Afebrile rate (mean age: < 2 years) | |||
| 620 (4 RCTs) | ⊕⊕◯◯a,b LOW | POR 0.91 (0.84 to 0.98) | Acetaminophen may slightly decrease afebrile rate |
| Afebrile rate (mean age: 2–5 years) | |||
| 1566 (9 RCTs) | ⊕⊕◯◯a,c LOW | POR 0.99 (0.97 to 1.01) | Acetaminophen does not decrease afebrile rate |
| Afebrile rate (mean age: > 5 years) | |||
| 353 (5 RCTs) | ⊕◯◯◯a,b,c VERY LOW | POR 1.01 (0.92 to 1.10) | Acetaminophen does not increase afebrile rate |
| Overall complication (mean age: < 2 years) | |||
| 382 (2 RCTs) | ⊕⊕◯◯b,c LOW | POR 0.96 (0.55 to 1.70) | Acetaminophen does not reduce overall complication rate |
| Overall complication (mean age: 2–5 years) | |||
| 2323 (11 RCTs) | ⊕⊕⊕◯a MODERATE | POR 0.99 (0.94 to 1.03) | Acetaminophen does not reduce overall complication rate |
| Overall complication (mean age: > 5 years) | |||
| 583 (5 RCTs) | ⊕⊕⊕◯b MODERATE | POR 0.71 (0.58 to 0.87) | Acetaminophen reduces overall complication rate |
Discussion
On the basis of the available evidence, the present pooled results revealed that children in the acetaminophen group displayed temperatures about 0.2°C higher than those in the ibuprofen group, which indicated that the efficacy of ibuprofen was slightly better than that of acetaminophen. However, this result might not be significant in clinical settings. By contrast, about 29% lower risk was observed in the acetaminophen group than in the ibuprofen group regarding overall adverse event rates in the subgroup of mean age of > 5 years. The safety finding may raise concerns in clinical practice.
The minimum age for using acetaminophen ranged from 0 months [46–48] to 2 months [49] and 3 months [6, 50]; however, the NICE guidelines provide no suggestions for age [51]. The dosage of acetaminophen varied as follows: a single dose of 10–15 or > 15 mg/kg, intervals between doses of 4, 4–6, or 6 h, and maximum daily dosage of 60–90 mg/kg/day.
Some studies have recommended 2 months [49], 3 months [50], and 6 months [6, 48] as the minimum age for ibuprofen administration, whereas no such suggestions are covered in other studies [46, 47, 51]. The dosage of ibuprofen is divergent in a single administration (5–10 or 10 mg/kg/dose), intervals between doses (6, 6–8 h), and a maximum daily dosage of 40 mg for all guidelines, except for the Italian Pediatric Society Guidelines, which allow the dose of up to 30 mg [47]. In the guidelines we discussed, all used either acetaminophen or ibuprofen in children with fever who appeared distressed. The choice of these two recommended antipyretics is made according to the child’s age, weight, and other characteristics. In some situations, ibuprofen administration should be cautious. Ibuprofen may worsen asthma symptoms and should be prescribed cautiously [50]. In children with dehydration, ibuprofen use is contraindicated by the Italian Pediatric Society Guidelines but recommended with caution by the American Academy of Pediatrics [6] and guidelines in South Africa and South Australia [46, 50]. In the case of varicella, using ibuprofen is contraindicated by the Italian Pediatric Society Guidelines but recommended with caution by the American Academy of Pediatrics and guidelines in South Africa [6, 47, 50]. Although part of our analysis supports these guidelines, some differences were noted.
According to our analysis, in children with a mean age < 2 years, ibuprofen was more effective than acetaminophen. The same result was observed in children with a mean age between 2 and 5 years. Moreover, the side effects compared between ibuprofen and acetaminophen demonstrated no obvious difference in groups with mean ages of under 2 years. Although ibuprofen may be preferred for a rapid antipyretic effect, more studies are required to establish its safety. In studies with a mean age of > 5 years, no obvious difference in effectiveness was observed between acetaminophen and ibuprofen. Furthermore, acetaminophen monotherapy demonstrated fewer side effects than did ibuprofen monotherapy. As per our observations, no differences were observed in the antipyretic effects between ibuprofen monotherapy and acetaminophen monotherapy with older age.
In total, 1,329 people comprised the acetaminophen group with available fever resolution data, and the acetaminophen group was administered dosages of 9.8, 10, 12.5, and ≥ 15 mg/kg (up to 30 mg/kg). We did not observe a notable increment in antipyretic effects with increasing dosage. Out of twenty-four included studies with the oral route of medication, there was only one study with the intravenous route of acetaminophen and one study with the rectal or oral route of acetaminophen and intravenous route of ibuprofen. There was a difference in pharmacokinetics when the administration route was different, which might have led to the variation of time-related antipyretic efficacy [52, 53]. Nevertheless, there was no outcome difference when these two studies with different routes of medication were weeded out from the analysis. Regarding the adverse effects of acetaminophen, our analysis demonstrated a trend wherein the risk of an adverse event increased, similar to that of the ibuprofen group, with an increasing acetaminophen dose. Multisystemic adverse effects caused by acetaminophen use have been mentioned in other studies including gastrointestinal (vomiting), dermatologic (skin rash), metabolic (hyponatremia), hematologic (pancytopenia), and hepatic (elevation of serum alkaline phosphatase and bilirubin). The incidence of adverse events mentioned earlier was still low, and few cases of catastrophic clinical outcomes caused by these adverse effects were reported [54, 55]. Therefore, we recommend a dosage of 10 mg/kg acetaminophen that exerts the maximum antipyretic effects with a relatively low adverse event risk. Compared with the acetaminophen group, a higher proportion of patients in the ibuprofen group developed adverse effects, especially symptoms of the gastrointestinal system. In the included studies, the gastrointestinal symptoms most documented were nausea, vomiting, abdominal pain, and diarrhea. By contrast, few studies have reported overt gastrointestinal bleeding symptoms, such as melena, hematemesis, and hematochezia, which indicated that there were not more significant events of gastrointestinal bleeding or mortality despite the higher risk of adverse effects in the ibuprofen group than in the acetaminophen group. Although the safety of ibuprofen raised little concern, subtle adverse effects attributed to medications still bother and affect the quality of life of the caregivers. Parents, who are not professionals of medicine or related fields, are extremely anxious about disease progression and adverse events caused by diseases in their children, even incurable illnesses such as the common cold [56]. Subtle adverse effects could conceivably worsen the parents’ quality of life.
Although the present study gathered evidence more comprehensively than did the previous systematic reviews, some methodological limitations existed. First, we could not stratify the diseases underlying the pyrexia, mainly because no data that could aid in distinguishing the underlying diseases were available. Interpreting the present pooled results cautiously and considering the underlying diseases before application in clinical practice is recommended. Second, acetaminophen and ibuprofen can be administered through various strategies. However, we could not draw conclusions on the optimal medication strategy because of insufficient evidence. Future studies should discuss this further to ensure optimal treatment outcomes.
In conclusion, the present evidence provides additional information on the effects of acetaminophen and ibuprofen monotherapies in febrile children, indicating that ibuprofen might be not superior to acetaminophen even in children with mean age of approximately 5 years. Moreover, acetaminophen monotherapy may be safer than ibuprofen monotherapy, particularly in children about 5 years old. In conclusion, as the efficacy and risk of adverse events are taken into consideration comprehensively, acetaminophen monotherapy might be a better choice for antipyretic purposes in children as compared with ibuprofen monotherapy.