In the past decade, tumor necrosis factor-α (TNF-α) antagonists have been used as the first-line treatment for autoimmune diseases, including rheumatoid arthritis, ankylosing spondylitis (AS), Crohn’s disease, uveitis, inflammatory bowel diseases, and psoriasis [1, 2]. In the 1970s, TNF-α was used as an anti-tumor drug by Lloyd Old et al. [3]. TNF-α was subsequently found to be a multifunctional cytokine with important roles in acute and chronic inflammation, antitumor responses, infection, and various disease processes, such as dyslipidemia, insulin resistance, and autoimmune diseases [4, 5]. Adalimumab has been approved by the United States Food and Drug Administration. The high affinity and specificity of adalimumab contribute to its safety and efficacy in clinical use [6]. Common adverse effects of adalimumab include infection, leukopenia, elevated creatinine phosphokinase and transaminases, headache, and skin rash [7]. Since adalimumab was approved for use in 2002, eosinophilia has been rarely reported following adalimumab treatment. The classification scheme of eosinophilia is as follows: mild, 0.5 × 109–1.5 × 109/l; moderate, 1.5 × 109–5.0×109/l; and severe, > 5.0 × 109/l [8]. Eosinophils directly cause cell damage by releasing specific granule contents, thereby damaging tissue integrity [9]. Thus, excess eosinophils exacerbate severe cell damage.
We describe a case of eosinophilia in a patient with prolonged administration of adalimumab to highlight the importance of assessing the blood count, particularly eosinophils, during administration of this biological agent. Further, we explore the use of disease-modifying antirheumatic drugs (DMARDs) for treating eosinophilia resulting from treatment with adalimumab.
A 17-year-old male who presented with a 1-year history of swelling and pain in both heels, accompanied by a 9-month history of pain in the back and right hip, was admitted to our hospital on July 17, 2014. On admission, physical examination revealed that his spine had an abnormal physiological curvature. Bipedal heel swelling and tenderness were noted. In specific tests, the bilateral straight leg elevation test was negative, Patrick’s test on the right was positive, pillow-wall distance was 5 cm, Schober’s test was 5 cm, and finger-to-ground distance was 30 cm. The muscle tone was normal, and bilateral tendon reflexes were elicited symmetrically without pathological reflexes.
Laboratory test results were as follows: human leukocyte antigen B27, positive; C-reactive protein, 15.6 mg/l; and erythrocyte sedimentation rate, 64 mm/h. Based on the comprehensive clinical assessment, laboratory tests, and imaging, the diagnosis of AS was established.
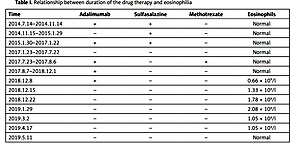
During hospitalization, the patient was administered adalimumab (40 mg) once every 2 weeks by subcutaneous injection after excluding relevant contraindications. Sulfasalazine tablets (1.0 g, twice per day) were administered orally along with doses of adalimumab. After 4 months of combination therapy with adalimumab and sulfasalazine, the patient’s symptoms were significantly relieved. The patient decided to discontinue treatment with adalimumab and continued with sulfasalazine tablets (1.0 g, twice per day) orally to control his condition.
On January 30, 2015, the patient experienced swelling and pain in both heels. Considering disease recurrence, adalimumab (40 mg) was administered once every 2 weeks. Sulfasalazine tablets (1.0 g, twice per day) were administered orally along with adalimumab. After 3 months, the patient’s condition improved. On January 23, 2017, sulfasalazine tablets and adalimumab were discontinued because of the patient’s low globulin level of 13.9 g/l. After 6 months, the patient’s globulin level returned to normal, and methotrexate tablets (10 mg, once per week) were administered orally. However, laboratory tests revealed an alanine aminotransferase level of 172 U/l and aspartate aminotransferase level of 68 U/l after administration of methotrexate tablets for 2 weeks. Thus, methotrexate tablets were immediately discontinued.
Subsequently, the patient was administered adalimumab (40 mg) only. After 1 week of treatment, routine blood tests showed an absolute eosinophil count of 0.66 × 109/l, leading to discontinuation of adalimumab. At this time, the patient had fatigue and discomfort. Eosinophilia was diagnosed through bone marrow tests at the Department of Hematology, Ningbo First Hospital. Bone marrow gene detection showed no myeloid-related pathogenic mutations. In addition, fluorescence in situ hybridization and qualitative detection of the ETV6-PDGFR and FIP1L1/PDGFRα fusion gene were all negative. Given that the eosinophilia was unexplained, the clinicians did not treat the conditions discussed. The eosinophilia persisted until May 11, 2019 (Table I).
Table I
Relationship between duration of the drug therapy and eosinophilia
Eosinophils, a type of human leukocyte, are extremely important in immune and allergic reactions. The physiological activity of eosinophils has been observed in multiple organs and tissues [10]. Eosinophils present antigens for killing bacteria and parasites and play key roles in inducing and maintaining chronic inflammation and tissue fibrosis [11]. Thus, the causes of eosinophilia are diverse. Hypereosinophilic syndrome is associated with multiple molecular defects in PCM1-JAK2, FGFR1, PDGFRα, and PDGFRβ. Tyrosine kinase activity is abnormally increased in these genetic mutations, leading to eosinophil overproduction. Differential diagnoses for eosinophilia include the primary considerations of hematologic malignancies, such as acute and chronic myeloid leukemia, and systemic mastocytosis. Eosinophilia can occur in inflammatory and immune diseases [12]. In allergies and infections, clonal or phenotypically abnormal T cells secrete the cytokine, IL-5, which can cause eosinophilia if present in excess amounts [13]. Pulmonary insufficiency and disseminated cryptococcosis are other factors associated with an increased eosinophil count [14].
Drug-induced eosinophilia is typically transient, mild, or even asymptomatic. In most cases, eosinophils return to normal levels in patients after drug withdrawal and those with severe symptoms recover quickly after symptomatic treatment. Few patients require corticosteroid treatment [15].
In this case, the patient was diagnosed with AS without a past history of asthma. The patient’s living environment was stable. The cotton bedding sheets at home and school were regularly cleaned. There were no reported environmental allergens, such as flowers or pollen, and food allergen test results were negative. Fatigue and discomfort occurred after eosinophilia. Environmental and dietary factors were screened, and the possibility of hematological diseases and malignant tumors were excluded (Table II A). Thus, drug-induced eosinophilia was highly suspected. The relevant drugs were not administered within half a year before the occurrence of eosinophilia; after discontinuing the drug, the eosinophil level gradually decreased and returned to normal levels. This effect was suspected to be related to adalimumab treatment. The causality term was ‘probable/likely’ using the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) scale (Table II B). The Naranjo Algorithm resulted in ‘probable’. In addition, we observed that the patient’s eosinophils continued to rise to 2.08 × 109/l a month after discontinuation of adalimumab. We speculate that this phenomenon was due to the fact that it took the patient a month to clear the remaining adalimumab from the body, given that the half-life of a single intravenous dose of adalimumab ranges from 15 to 19 days [16].
Table II
A – Screening for causes of eosinophilia. B – WHO-UMC causality categories of the case
Although cases are rare, treatment with TNF-α antagonists has been reported to be associated with eosinophilia in clinical scenarios [17]. These cases suggest the involvement of unknown mechanisms that must be explored. Compared with previous cases, our case was unique because the increase in eosinophils did not occur immediately after adalimumab administration, but rather only after the patient discontinued methotrexate or sulfasalazine because of his medical condition. When methotrexate or sulfasalazine was combined with adalimumab, the patient did not develop eosinophilia. The reasons for using combination drug therapy were as follows: first, considering the poor prognosis of the patient, DMARDs were added to the adalimumab treatment; and second, considering the cost of the biological agent, we planned to reduce the use of adalimumab in later stages. Methotrexate is a folic acid antagonist that can reduce the activity of adenosine synthetase, inhibit the synthesis of DNA, and plays an anti-inflammatory and immunosuppressive role. Currently, methotrexate is commonly used to treat rheumatism. Sulfasalazine is a sulfanilamide drug with antibacterial, anti-inflammatory, and anti-immune properties, and is recognized as a DMARD for treating rheumatoid arthritis. Methotrexate has been reported to cause pancytopenia and inhibit the synthesis of new purines in T lymphocytes, thus, affecting IL-5 levels [18]. Sulfasalazine reduces eosinophil activation. Both drugs may reduce the eosinophil count. Based on the medical history, the eosinophil-lowering effects of DMARDs may have been eventually offset by the adverse effects of adalimumab. Therefore, eosinophilia only appeared after discontinuation of combination therapy. However, no relevant reports exist to support this hypothesis, which should be further evaluated in basic research.
AS is a chronic and complex autoimmune disease affecting the spine and sacroiliac joints and has a prevalence of approximately 0.24% in the Chinese population. The high disability rate greatly affects the physical and mental health of patients. Presently, there is no cure for AS, and patients often require lifelong medication. DMARDs are used regularly in AS to treat several inflammatory conditions; however, they appear to have little or no effect on patients with axial AS. Thus, the emergence of anti-TNF drugs gives patients hope. Evidence suggests that anti-TNFs are the most effective drugs for treating AS. However, because of its high cost and adverse reactions, some patients must discontinue adalimumab treatment, despite its good therapeutic effects.
In conclusion, this case provides insight into the combined use of DMARDs and adalimumab, which can lower costs and leads to fewer adverse reactions. Moreover, we reported this case to improve our understanding of adverse reactions related to adalimumab. This rare case provides a foundation for further treatment options for AS.