Introduction
Short stature is defined as a condition in which the height of the child is below –2.0 SDS from mean values for a given age, sex and population [1]. If no identifiable conditions of short stature (normal birth weight, no evidence of chronic organic diseases and endocrine diseases, no dysmorphic features or skeletal dysplasias, no psychiatric disorder or severe emotional disturbance, normal food intake) are recognized, idiopathic short stature (ISS) is diagnosed [1, 2]. The ISS children are usually subjected only to follow-up. However, in many cases, patients do not reach the normal final height, as the causes of short stature remain unknown. Recently, there has been a discussion about growth hormone (GH) therapy in children with ISS (non-GH-deficiency) [3–5]. In addition to safety and efficiency, the psychological aspects and costs are taken into account [6–8]. The best results are seen in children with low insulin-like growth factor I (IGF-I) [9–11].
Gastrointestinal tract diseases (GIDs) are among the factors which can affect the growth rate in children, as malnutrition is one of the causes of secondary IGF-I deficiency [1]. The following GIDs should be mentioned here: celiac disease (CD) [12], inflammatory bowel disease (IBD) [13], lactose malabsorption (LM) [14], cystic fibrosis (CF) [15], Helicobacter pylori (HP) infection [16], Ascaris sp. (Asc) infection [17], and Candida albicans (Calb) colonization [18]. In most of these GIDs, the presentation is characteristic and the diagnostics is not difficult. However, in some cases, the slow growth rate may precede other signs of GIDs or the latter may be scarce, uncharacteristic or poorly expressed (oligosymptomatic GIDs). The frequency of GIDs caused by microbiota or induced by autoimmune processes is continuously increasing all over the world.
Thus, the aim of the study was to evaluate the frequency of unexpected, oligosymptomatic GIDs in children with ISS and to assess the influence of individual GIDs on children’s height and body mass in comparison to short children without any GIDs, as well as in comparison to a group of healthy, normal height children.
Material and methods
The study group included 101 children with ISS (43 girls and 58 boys), aged 5.5–14.5 years (mean ± SD: 10.37 ±3.28 years). The body height was measured using a stadiometer and the height standard deviation score (HSDS) was calculated according to current population standards. Only children with HSDS below –2.0 were included in the study group. Next, based on the child’s position on percentile charts, the height age (HA) was calculated (as the age given for the 50th percentile for the child’s height). The body mass was assessed in all patients, followed by determining the body mass index standard deviation score used for HA (BMI SDS for HA). The parameter in question reflects the nutrition state of short children better than BMI SDS calculated for calendar age. None of the children reported symptoms from the GI tract or suffered from chronic cardiovascular, respiratory or urinary system diseases. None of them had been previously diagnosed and treated for GIDs. Children with dysmorphic features, those suspected of skeletal dysplasia and girls with Turner’s syndrome were excluded from the study. In children with hypothyroidism, GH and IGF-I secretion was evaluated after the concentrations of TSH and thyroxin were normalized by applying an appropriate substitution treatment. The control group included 94 healthy, normal height children (57 girls and 37 boys), aged 3.5–16.6 years (mean ± SD: 10.61 ±3.48 years).
Hormonal assessment
In each short child, a 3-hour, nocturnal profile of GH secretion was recorded every 0.5 h, starting from the 1st h after falling asleep. Next, two stimulation tests were performed on subsequent days of hospitalization: the oral clonidine test with the dose of 0.15 mg/m2 and GH measurements at time 0 and at the 30th, 60th, 90th and 120th min of the test. The second stimulation test involved intramuscular administration of glucagon in the dose of 30 μg/kg, with GH measurements at time 0 and at the 90th, 120th, 150th and 180th min. Peak GH concentration (GHmax) was determined in both tests and after falling asleep. Only patients with a GHmax value ≥ 10 ng/ml were included in the study group. In each child, fasting serum concentrations of IGF-I were measured. For comparison, among children of different age and sex, IGF-I concentrations were expressed as IGF-I SDS for HA, according to reference data [19]. In the normal height children from the controls, GH and IGF-I assessment was not performed.
The immunometric method was used to measure GH and IGF-I serum concentrations with Immulite, DPC assay sets, for GH: with sensitivity: 0.01 ng/ml, range up to 40 ng/ml, the conversion index: ng/ml × 2.6 = mIU/l, the intra-assay CV: 5.3–6.5% and inter-assay CV: 5.5–6.2%, and for IGF-I: with sensitivity: 20 ng/ml, range up to 1600 ng/ml, intra-assay CV: 3.1–4.3% and inter-assay CV: 5.8–8.4%.
Gastrointestinal assessment
Gastrointestinal assessment was performed in each child included in the study groups (children with short stature and controls).
In order to diagnose CD, first, a serologic test was used: after excluding IgA deficiency phenotype, the tissue antitransglutaminase immunoglobulin A antibodies (tTG-IgA-Abs) were searched for in patients. Next, in all patients with a positive serological test, CD was confirmed by specific histological characteristics of villous atrophy, according to the modified Marsh-Oberhuber criteria [20, 21] during standard endoscopy with biopsy and next by the genetic assessment (DQ2DQ8).
To diagnose IBD, serological markers were investigated: the perinuclear anti-cytoplasmic antibody (pANCA), Saccharomyces cerevisiae IgA and IgG antibodies (ASCA), anti-Escherichia coli outer membrane porin (OmpC) antibodies and Pseudomonas fluorescens IgA antibody (I2). Calprotectin and a fecal occult blood test (FOBT) were also used in the diagnostic process as fecal markers of IBD. If IBD was suspected, the colonoscopy procedure with mucosal biopsies was recommended.
In order to diagnose LM, the hydrogen breath test (HBT) was performed. For diagnostic purposes, 0.5–1 g lactose/kg body mass in a water solution was administered (max. 25 g) after an overnight fast. Next, mucosal biopsy in conjunction with upper GI endoscopy was performed to assess lactase activity.
In order to diagnose CF, the quantitative pilocarpine iontophoresis test (QPIT) was run, collecting sweat and performing a chemical analysis of its chloride content. A value of over 60 mmol/l is consistent with the diagnosis of CF.
Helicobacter pylori infection was diagnosed based on positive results of the rapid urease test during upper GI endoscopy. However, serology tests were also performed which assessed the quantitation of IgA and IgG antibodies against HP by means of an enzyme-linked immunosorbent assay. It was valuable for the attempt to estimate the usefulness of this non-invasive method in diagnostics of short stature in children in which any GI symptoms were not observed (i.e. in children without recommendations for GI endoscopy).
To diagnose Ascariasis sp. infection, the serology test was performed.
In order to diagnose Candida albicans colonization, stool samples from patients were cultured for Candida sp., but only significant levels of Candida albicans were taken into consideration as candidiasis mucosae of GI. In all children, gastrofiberoscopy was performed to confirm the data from screening tools.
Statistical analysis
The Shapiro-Wilk test was used to assess distribution of the variables. The χ2 test and a one-way ANOVA were applied for statistical analysis, with the subsequent use of a post-hoc test, in order to statistically assess differences among groups. Correlations were evaluated using Pearson’s test. Statistically significant differences were accepted when p-value was below 0.05.
Ethical approval was granted by the Bioethical Committee at the Polish Mother’s Memorial Hospital – Research Institute in Lodz, Poland.
Results
The data of children from the ISS and control group are presented in Table I. It was not surprising that ISS children were significantly shorter than children from the control group. However, it is worth noting that the mean value of the body mass (expressed by BMI SDS for HA) was statistically lower in children with ISS than in the controls. In children with ISS, in addition to normal GH concentration, the IGF-I concentration (expressed by IGF-I SDS for HA) was below the normal range (Table I).
Table I
Data of children with idiopathic short stature (ISS) in comparison to healthy, normal height children (control group)
Among the analyzed cohort of 101 children with ISS, in 76 (75.2%), one or more GIDs were diagnosed, with the highest frequency of: Calb colonization (46.5%), LM (33.7%), HP (24.7%) and/or Asc (21.8%) infections. Thus, only in 25 children (24.8%) were no GIDs found.
In the controls, GIDs were detected with a significantly lower frequency – in only 28 children (29.8%). The frequency of particular GIDs in the group of ISS children and in the controls is presented in Table II.
Table II
Frequency of individual gastrointestinal diseases (GIDs) observed in children with idiopathic short stature (ISS) in comparison with healthy, normal height children (control group)
[i] Additional data
In our group of ISS children, an increased concentration of tTG-IgA-Abs was detected in 6 of them (5.9%). Immunoglobulin A deficiency was confirmed in 8 (6.6%) children; in all of them the levels of tissue anti-transglutaminase IgG antibodies (tTG-IgG-Abs) were normal. In all children with increased concentrations of tTG-IgA-Abs, we confirmed CD on the basis of the result of intestinal biopsy and intestinal villi pathology assessment.
The fecal occult blood test was positive in 5 children – 2 with CD, 1 with IBD and 2 with HP; in all of them the stool calprotectin was normal and ANCA and ASCA concentrations were not enhanced. In a boy with IBD, both HP infection and Calb colonization were additionally observed. ANA, pANCA, ASCA and I2 were normal in all children. In one boy we observed high concentrations of OmpC, but without other clinical signs of IBD (colonoscopy was not performed in him).
In the analyzed group of children, LM was confirmed in 34 (33.7%) cases, based on HBT. In all of them, absence of the lactase enzyme in conjunction with upper GI endoscopy was noted. However, we identified many cases with absence of the lactase enzyme in conjunction with upper GI endoscopy without a positive result of HBT (false positive results).
The diagnosis of HP infection was detected on the basis of the urease test in 25 (24.7%) children. In all of them, chronic gastritis was confirmed. Moreover, in 4 of them, villous atrophy 3a according to the Marsh classification was observed. It should be noted that increased levels of IgG antibodies against HP in 18 and of IgA in only 14 children were observed. Thus, in all children with positive serological results, the urease test confirmed acute HP infection, but in 8 other children, HP infection was confirmed by the urease test and histopathology while the serological test was negative. In these children, the results of the serological test were falsely negative.
In about 38.6% of short children with GIDs, only one of the GIDs mentioned above was diagnosed, but in the rest, more than one GID was confirmed: in 28.9% of children – 2, in 7.4% – 3 and in 1.7% – 4 GIDs. It was significantly higher than in the controls: in only 16.0% of children – 1 GID, in 5.3 % – 2 GIDs and in 2.1% – 3 GIDs.
Furthermore, to assess the influence of a particular type of GID on the nutritional status and height deficiency in the child, we compared the auxological data and GH and IGF-I secretion in ISS children with only one type of GID each. We found that the children with HP and Calb tended to be slimmer and shorter than children with LM and Asc. Moreover, in children with HP, the values of IGF-I SDS were the lowest among the groups, significantly lower than in children without any GIDs. The data are presented in Table III.
Table III
Auxological and hormonal data in idiopathic short stature (ISS) children without any GIDs and with only one type of analyzed GID depending on the kind of disease
| Parameter | No GIDs | Lactose malabsorption | Helicobacter pylori | Candida albicans | Ascaris sp. | Analysis of variance p-value |
|---|---|---|---|---|---|---|
| Number of children | 24 | 12 | 9 | 10 | 8 | |
| Age [years] | 9.91 ±3.93 | 10.17 ±3.49 | 8.87 ±3.66 | 10.26 ±3.78 | 9.75 ±3.01 | NS |
| Height [cm] | 124.21 ±19.81 | 124.85 ±16.19 | 120.20 ±15.79 | 127.66 ±18.47 | 127.13 ±14.56 | NS |
| Height SDS (HSDS) | –2.10 ±1.21 | –2.15 ±0.37 | –2.36 ±0.55 | –2.48 ±0.82 | –2.12 ±0.86 | NS |
| Body mass [kg] | 25.65 ±11.98 | 25.45 ±8.23 | 22.00 ±7.34 | 25.54 ±8.70 | 27.25 ±9.30 | NS |
| Body mass index [kg/m2] | 15.78 ±2.68 | 15.90 ±2.25 | 14.82 ±1.17 | 15.16 ±1.70 | 16.41 ±3.03 | NS |
| BMI SDS for HA | –0.19 ±1.04 | –0.04 ±0.90 | –0.47 ±0.32 | –0.46 ±0.72 | –0.01 ±1.23 | NS |
| GH max [ng/ml] | 21.64 ±10.66 | 20.85 ±9.87 | 30.90 ±13.77 | 19.63 ±7.42 | 18.38 ±7.22 | NS |
| IGF-I SDS for HA | –0.82 ±0.87a | –1.15 ±1.02 | –1.33 ±0.84a | –1.12 ±0.72 | –0.84 ±0.70 | < 0.05 |
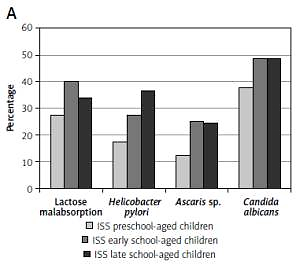
The frequency of GIDs was assessed in three age groups: preschool-aged children (4–7 years), early school-aged children (8–11 years) and late school-aged children (12–16 years), separately in children with short stature and in the controls. We observed that the frequency of GIDs is higher in older than in younger children in both groups; the results are presented in Figure 1.
Figure 1
Frequency of referred gastrointestinal diseases (Helicobacter pylori infection, lactase deficiency, Ascaris infection and/or Candida albicans colonization) in particular age groups: preschool-aged children (4–7 years), early school-aged children (8–11 years) and late school-aged children (12–16 years) in analyzed group of short children (A) and in control group (B)
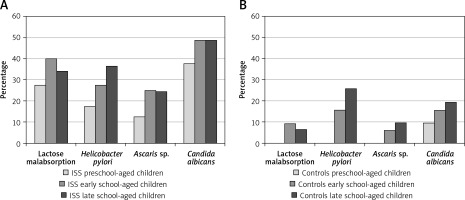
Despite that, we did not observe any differences with regard to BMI SDS for HA among the three age groups of children with short stature, while HSDS and IGF-I SDS values were significantly lower in the group of the oldest children than in the group of the youngest ones (Table IV).
Table IV
Auxological and hormonal data of short children sorted into particular age groups: preschool-aged children (4–7 years), early school-aged children (8–11 years) and late school-aged children (12–16 years)
| ISS group | Preschool-aged children (4–7 years) n = 40 | Early school-aged children (8–11 years) n = 40 | Late school-aged children (12–16 years) n = 41 |
|---|---|---|---|
| Gender female/male | 16/24 | 20/20 | 17/24 |
| Age [years] | 6.26 ±1.03a,b | 10.40 ±1.06b,c | 13.80 ±1.49a,c |
| Height [cm] | 107.40 ±6.63a,b | 127.40 ±7.05b,c | 143.21 ±8.88a,c |
| Height SDS (HSDS) | –2.12 ±0.82a | –2.31 ±0.84 | –2.69 ±1.01a |
| Body mass [kg] | 17.20 ±3.17a,b | 25.89 ±5.37b,c | 35.17 ±8.05a,c |
| Body mass index [kg/m2] | 14.83 ±1.62 | 15.83 ±2.40 | 16.98 ±2.48 |
| BMI SDS for HA | –0.27 ±1.03 | 0.01 ±1.17 | 0.01 ±0.85 |
| GH max [ng/ml] | 20.55 ±9.92 | 17.68 ±10.05 | 19.27 ±9.65 |
| IGF-I SDS for HA | –0.63 ±0.78a,d | –1.25 ±1.17d | –1.48 ±1.24a |
Discussion
Our study proved that GIDs are present in most children from the analyzed group of short children, in whom ISS had been previously diagnosed. The question was whether the frequency of oligosymptomatic GIDs is higher in children with short stature than in the general population and, therefore, whether the listed GIDs can be the causes of short stature.
Firstly, we found a significantly higher frequency of most analyzed GIDs in ISS children than in children from the controls, particularly as regards CD, LM, HP and Asc infections and Calb colonization. We should, however, be aware of the relatively small number of children in the control group for this type of comparison. Therefore, in the analysis, we also referred to the data available in the literature for the Polish population and for Central and Eastern Europe.
Celiac disease occurs in 1–3% of children, in many of whom a “silent” CD is documented [22]; impaired growth is observed in about 1/3 of cases [23, 24]. We confirmed other authors’ observation that in children with short stature, the frequency of CD is higher than in the general population: 6% vs. 1–3% [12, 23], although in our controls not even one such case was found. Untreated CD leads to inhibition of body weight gain and growth rate due to both malabsorption and the intestinal escape of proteins [12]. Thus, we agree with Bhadada et al. that all short children should be screened for CD, irrespective of GI symptoms [25].
In the group of short children we analyzed, the most frequent GID was the colonization of Candida albicans. Candida organisms commonly colonize the human GI tract as a component of the resident microbiota. Their presence is generally benign. Recent studies, however, show that a high level of Candida colonization is associated with several diseases of the GI tract [18]. We diagnosed colonization of Candida albicans in more than 45% of short children. This frequency is still lower than in other Polish studies – e.g., in Macura and Witalis’ study, fungal colonization was detected in 61.5% of the tested children’s specimens [26]. It should be stressed that in our study only significant levels of Candida albicans were taken into consideration. However, in the control group, the colonization of Candida albicans was observed only in 15%. It should be noted that the children with Candida albicans colonization were slimmer than the other ones. The impact of this pathogen on the growing process in children should be a subject of further studies. It seems that fungal colonization of the GI tract may lead to disturbances in the development of the child [18] and dysregulation of the appetite and growth process, probably in the mechanism of molecular mimicry with some neuropeptides [27]. In the molecular mimicry phenomenon mechanism, peptide fragments identical to leptin, ghrelin, neuropeptide Y, orexin and α-melanocyte stimulating hormone (α-MSH) were found in proteins belonging to Candida albicans species [27]. We can speculate that the cross-reactions between antibodies and neuropeptides involved in the regulation of growth and food intake may disturb the normal development of the child.
The second most common GID was LM. Primary adult-type hypolactasia (or lactose malabsorption) is an autosomal recessive condition resulting from the physiological decline of lactase enzyme activity in the intestinal cells. It occurs in a large proportion of individuals and appears at birth or later (in 18% of children before the age of 10) [28]. Secondary hypolactasia is a result of secondary damage of the enterocytes (post-infectious, drug-induced, allergic, inflammatory background, celiac disease) and leads to transient lactase deficiency. Although patients with LM are often unaware of their illness, it has been proved that non-milk drinkers are lighter and significantly shorter than milk drinkers [14]. In our study group, LM was found in 33.7% of short children, which was a higher frequency than in controls (only 6.4%) as well as in the data for the general population [29]. It is well known that the frequency of this condition varies, depending on ethnicity, with reported lower prevalence in Northern Europe (< 5%), compared to Southern Europe (70–80%) [30]. Moreover, the onset of adult-type hypolactasia is related to age: lactase activity is highest at birth and declines after weaning. In our study, it was observed that the frequency of LM was higher in the older than in the younger children. In 2005, Gugatschka et al. showed that an individual’s knowledge of their LM personal status was very poor and the correlation between characteristic genotypes, self-reported LM and milk drinkers’ habits was limited [14]. On the other hand, non-milk drinkers were smaller and slimmer and the body mass density was lower. In our study, we did not take into account whether the child was a milk drinker or not, so it is difficult to refer to this issue. However, at the beginning of the study, when we asked about complaints and chronic diseases, milk intolerance was not reported. Nevertheless, it was found that the height deficiency in children with LM was similar to that in children with other diseases of the gastrointestinal tract, and therefore LM may be a potential cause of the child’s worse growth.
Helicobacter pylori infection in children is expressed in a variety of clinical manifestations – from asymptomatic to organic diseases of the GI tract. It may cause chronic inflammation of the lining of the duodenum with impaired intestinal digestion and absorption, which affects the child’s development [16]. It is estimated that HP infections in Poland are confirmed in 30% of patients living in a rural environment and in about 10% coming from an urban environment [31]. In our controls, we observed HP infection in 13% of children, similar to the data for an urban environment, mentioned above. Thus, we noted a significantly higher frequency (about 25%) of HP infection in children with short stature in comparison to controls. In other studies, also a higher frequency of HP in ISS than in control children was reported [32]. Fialho et al. recently reported that children with HP infection are shorter than children without it [33]. It seems that this infection is an important risk factor of being shorter [34]. However, the mechanism is not clear; it may be connected with lower ghrelin production [35] or cross-reaction with same neuropeptides in the molecular mimicry phenomenon mechanism (see above) [27]. Moreover, it is known that the growth of children is improved after successful eradication [36]. However, one should be aware that, according to ESPGHAN [37], the preferred diagnostic method for the diagnosis of HP infection is endoscopy of the upper gastrointestinal tract with the rapid urease test, supplemented with a histological evaluation of the collected mucosa sections, but not serological tests. In our group of children, the diagnosis of HP infection was detected on the basis of positive results of the rapid urease test and presence HP cultures during GI biopsy. Serological tests proved to be false negative in 1/3 of cases (Table III), and this must be taken into account when carrying out diagnostics.
Ascariasis is still a current pediatric clinical problem, characterized by non-specific clinical manifestations. In Poland, Ascaris-positive preschool-aged children (4–7 years) accounted for 8.1% and school-aged children (8–18 years) for 15.8% of the cases [38]. Large numbers of adult worms in the small intestine can cause abdominal pain, obstructive ileus, malnutrition and growth failure. However, in most cases, this infection remains asymptomatic until the number of worms in the intestine considerably increases [17]. However, malnutrition and growth failure may be observed at an earlier stage of the infection. We observed a higher frequency of Ascariasis in our group of short children than in controls or in Wasilewska’s et al. study [38]. Recently, preventive treatment has been proposed in order to reduce the frequency of Ascariasis in children [17].
The frequency of IBD (ulcerative colitis or Crohn’s disease) in Europe is estimated at about 1–3 in every 10,000 children [13]. The clinical symptoms are usually very characteristic if the disease is advanced, even though the first symptoms may be scarce. It is estimated that in 6–12% of cases, the first symptoms may be connected with weight gain inhibition and/or a slow growth process [39]. While searching for GIDs in asymptomatic (except short stature) children, we found 1 case of IBD.
Despite the fact that in some cases of CF a slow growth rate and short final height are observed [15] we did not note any case of CF in our study group.
In children with ISS, there was no difference in nutritional state, regardless of the lack or presence of one or more GIDs. Thus, in our opinion, the nutritional state should not be the deciding factor in starting or abandoning the search for GIDs in children with short stature.
It was very interesting that in ISS children, the values of IGF-I concentration (expressed by IGF-I SDS) were reduced to similar degree. It is generally accepted that IGF-I is a peripheral mediator of GH action on the tissues and it is formed and secreted under the influence of GH. Therefore, another factor (other than GH deficiency) must have exerted a decreasing effect on IGF-I concentration. HP infection is certainly one of the factors in question, because in children with ISS bearing HP infection, IGF-I concentrations were significantly lower than in ISS children without GIDs. The reason for the low IGF-I concentration in the ISS group without GIDs and with other GIDs is not explained. It is possible that malnutrition due to different mechanisms causing the lowering of IGF-I synthesis is one of the factors with the important role of sirtuin action [40]. The next factor influencing the reduction of IGF-I expression in rats is damage to the function of the pancreas (diabetes) [41].
Regardless of these considerations, we found that in short children, the degree of growth deficiency and value of IGF-I SDS were the worst in the group of the oldest children. It indicates the need for an early diagnosis and immediate medical treatment in short children with GIDs. This is important also due to the growing difficulty in eradication of HP as a result of antibiotic resistance [42], which increases with the duration of infection. It should be emphasized that HP is a risk factor for gastric cancer and gastric MALT lymphoma.
In conclusion, in children with ISS, a high frequency of oligosymptomatic GIDs that may affect the growth rate and final height was observed. It points to the need to search for the gastrointestinal causes of short stature in each short child. The diagnostic tools should include a blood sample for the tissue antitransglutaminase immunoglobulin A antibodies (after excluding the IgA deficiency phenotype), HP and Asc antibodies, hydrogen breath test, as well as a stool sample used for the occult blood test, the calprotectin concentration test and culture for Candida sp. However, HP antibodies are not enough to detect all the cases with HP infection.
It should be taken into account that the proper diagnosis and treatment of GIDs in a child may restore the normal growth rate and protect the child against unnecessary long-term treatment with daily GH injection. The pathomechanisms responsible for short stature in these cases may be different, although it seems that the reduced production of IGF-I plays an important role.