Introduction
The thymus is an organ that develops mainly from endoderm from the 3rd and 4th pharyngeal pouches during embryogenesis. It is composed of the inner medulla and peripheral cortex and it is surrounded by an outer capsule. It starts to develop around the 9th week of human development and it is fully developed by birth. The thymus continues to grow between birth and puberty and then begins to involute through atrophy. It may also undergo dynamic changes during a state of illness. It constitutes a central (primary) lymphoid organ that controls the early development of peripheral (secondary) lymphatics and cellular immunological competence of immunologic cells, which provides resilience throughout the time of fetal, neonatal and child development. The thymus is a lymphoid organ, where T lymphocytes are promoted through a positive and a negative selection process to mature T lymphocytes that carry an array of different surface T-cell antigen receptors. T cells leave the thymus in their naive form, i.e. when they have not encountered their cognate antigen yet, and occupy peripheral tissues where they differentiate into effector and/or memory cells after stimulation with antigen [1].
The fetal thymus may be visualized using an ultrasound machine and is typically located in the mediastinum anterior to the great arteries and superior vena cava. In the past years, the size of the fetal thymus has served not only as a marker of genetic or heart defects but also as a predictive factor for intrauterine growth restriction (IUGR), premature birth, preeclampsia, chorioamnionitis or even neonatal sepsis [2–6]. Fetal thymus evaluation has become an important diagnostic and predictive tool for obstetricians, neonatologists and pediatric immunologists that may predict immunodeficiencies and qualify for further vaccination protocol. Consequently, a need has arisen to define and provide ultrasound nomograms of the fetal thymus, which could provide a very useful point of reference like in the case of various growth charts for neonates and children used in doctors’ everyday practice.
Material and methods
A total of 410 fetuses were qualified for the study and evaluated at the Prenatal Cardiology Department of the Polish Mother’s Memorial Hospital Research Institute the between the years 2016 and 2018. Fetuses with heart defects were excluded from the study. Other anatomical defects or anomalies such as intrauterine growth restriction (Intrauterine growth restriction (IUGR) refers to a condition in which a fetus is unable to achieve its genetically determined potential size. This functional definition seeks to identify a population of fetuses at risk for modifiable but otherwise poor outcomes.) and macrosomia (Fetal macrosomia: the term macrosomia is used to describe a newborn with an excessive birth weight. Fetal macrosomia has been defined in several different ways, including birth weight greater than 4000–4500 g or greater than 90% for gestational age.) were also disqualifying. Patients with diabetes, obesity and autoimmune diseases, patients receiving preparations of heparin, acetylsalicylic acid and steroids and thyroid hormones were also excluded from the study group. Further exclusion criteria consisted of any systemic functional change that could be linked with infection, such as tricuspid valve insufficiency, idiopathic oligohydramnios (Oligohydramnios: refers to amniotic fluid volume that is less than expected for gestational age. It is typically diagnosed by ultrasound examination and may be described qualitatively (e.g., normal, reduced) or quantitatively (e.g., amniotic fluid index (AFI) ≤ 5)) or polyhydramnios (Polyhydramnios: is an abnormal increase in the volume of amniotic fluid. In singleton pregnancies it is defined as either the deepest vertical pocket of ≥ 8 cm or an amniotic fluid index of ≥ 24 cm.), hydronephrosis, etc.
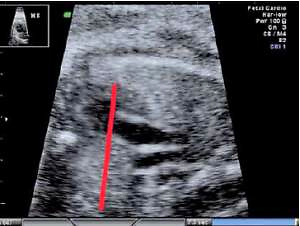
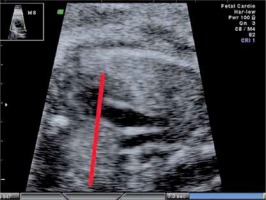
The fetal thymus was evaluated with transabdominal ultrasonography between the 14th and 40th week of gestation during a standard echocardiography examination performed routinely at the Prenatal Cardiology Department of the Polish Mother’s Memorial Hospital Research Institute. The plane used for evaluation visualizes the thymus immediately posterior to the sternum at the level of three great vessels of the mediastinum: the main pulmonary artery with its right and left bifurcation (MPA with RPA and LPA), the aorta (Ao) and the superior vena cava (SVC) (Figure 1). After obtaining a standard transverse view encompassing the three great vessels thymus measurements were attempted, i.e. maximal transverse diameter (mm), circumference (mm) and surface area (cm2). The maximum transverse diameter was defined by a line drawn perpendicularly to the line between the middle of the sternum and the vertebral column at the level of the thymus-lungs interface. The circumference was measured by tracing the transverse outline of the thymus immediately behind the sternum anteriorly and towards the interface with lungs on the sides, across the three great vessels of the mediastinum posteriorly. The methodology used has been described in other studies [7, 8].
Statistical analysis
Linear regression was used for statistical analysis, yielding 3 models, each with a different dependent variable (thymus maximal transverse diameter, circumference and area) and with gestational age (weeks) as the independent variable. The confidence interval for each model was set at 80% to aid the comparison with centile grid growth charts for neonates and children. For instance, since a 90–97th centile for a child mass indicates overweight and a 3–10th centile indicates underweight, the normal weight is accepted to be between the 10th and the 90th centile. Thus, the confidence interval was set at 80% by analogy (90th centile minus 10th centile = 80). The test was regarded as statistically significant when p < 0.05. The statistical analysis was performed using SPSS v.25.
Results
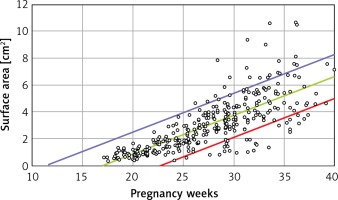
From a total of 410 fetuses the thymus transverse diameter, circumference and area were successfully measured in 410, 320 and 330 cases, respectively. The probabilities are lower than 0.0005 for each model, which means that each model is quite statistically significant and that the coefficient of determination R 2 is above, each being statistically significant.
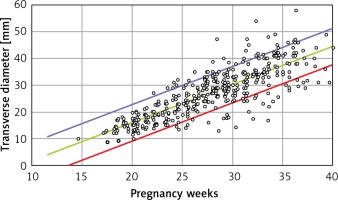
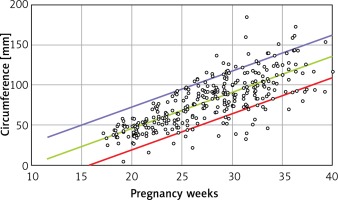
Linear formulae: Transverse diameter (mm) = – 12.478 + 1.424 × gestational age (weeks). Circumference (mm) = –39.382 + 4.272 × gestational age (weeks). Area (cm2) = –4.717 + 0.280 × gestational age (weeks) (Tables I and II).
Table I
Probabilities for each model of thymic measurements
Table II
Mean thymic parameters, 80% confidence interval and standard deviation
R2 is the coefficient of determination. The interpretation of R2: the percentage of variance of the endogenous variable (thymus parameter) that can be explained by the variance of the exogenous variable (gestation age). Its maximal value equals 1 when all points lie on the linear regression line. P for R2 is the probability in hypothesis testing 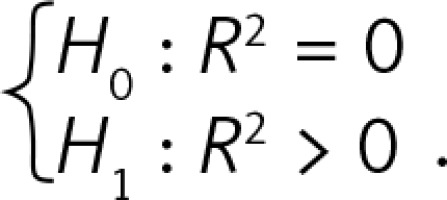 .
.
B is the regression coefficient. A simple linear regression model can be presented as Y = A + B × X, where Y is the thymus parameter, X is the gestation age, A is the constant and B is the regression coefficient. P for B is the probability in hypothesis testing: 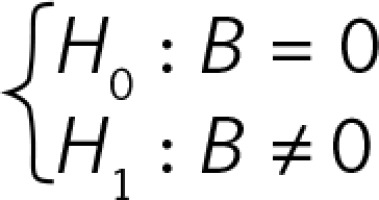 (Figures 2–4).
(Figures 2–4).
Discussion
The aim of this study was to determine the feasibility of USG examination in developing a model of fetal thymus evaluation and to develop thymic nomograms of healthy fetuses. The process of obtaining the thymic maximal transverse diameter is a marginal addition to the echocardiography examination since it is visualized together with the desired three great vessels of the mediastinum. Also, as in our case, it is often an inherent element of the USG fetal examination protocol. Since the outline of the fetal thymus may vary, the manual tracing of the thymic transverse circumference and the surface area may be unreliable and unfit when visualizing the three great vessels of the mediastinum.
This is the reason why the number of measured circumferences and areas is different from the number of measured transverse diameters. Namely, after having reviewed the available USG scans that yielded a successful measurement of the thymic maximal transverse diameter, only 320 and 330 were further qualified for subsequent circumference and area measurement, respectively.
Functional disorders in otherwise healthy fetuses (oligo- or polyhydramnios, heart valve insufficiency, arrhythmia, pericardial effusion) or in mothers (flu symptoms, urinary tract infection) led to exclusion from the study because they often correlate with potential intrauterine infection. Hamamoto et al. pointed out that amniotic fluid sludge and a small thymus may be signs of intraamniotic infection and preterm labor [9]. Similarly, the study of Yinon et al. found that a smaller fetal thymus correlates with chorioamnionitis and with the resulting preterm premature rupture of membranes [10]. When the thymic size is evaluated postnatally, it can be used to predict the likelihood of survival in preterm infants. This was concluded from the study of Tooke et al., who found that cardiothymic/thoracic ratio in preterm neonates correlated with survival outcome (p = 0.029) [11]. Moreover, a correlation with pre-eclampsia and infection was also found but it was significant only in the first 24 h of life when clinical signs of infection are yet to develop. Evidence of an association between thymic size and infection is further provided by a pathology study of Galvina-Durov et al., who analyzed post-mortem a total of 100 premature neonates and found that infection as a cause of death was connected with advanced thymic involution (p < 0.001) [12]. Consequently, it is agreed that the size and function of thymus depend strictly on the accompanying infection as well as on external factors, such as smoking or chronic steroid therapy during pregnancy [13–15]. It was estimated that the thymus may shrink by up to 40% of its original size [16] and that environmental or perinatal factors may be associated with the risk of developing an autoimmune disease [17].
Because of the contemporary rise of diseases of affluence, particularly autoimmune diseases, there is an increase in the number of patients treated chronically with steroids. A long history of steroid therapy is observed not only in internal medicine patients with asthma, kidney insufficiency, rheumatism, thrombocytopenia or flares of inflammable bowel disease but also in pregnant patients with recurrent miscarriages linked to antiphospholipid syndrome or in vitro fertilization [18].
Also short treatment with steroids remains significant when administered perinatally [14]. Researchers from Hamburg Medical University showed that betamethasone administration leads to thymus shrinkage and loss of thymocytes, which are observed for up to 3 days after steroid administration [19]. The enlargement of the thymus, although less frequent, is also to be considered. Transient hyperplasia may be observed in fetuses or in adults who recover from stress such as infection, steroid therapy, radio or chemotherapy, surgery or burns. Yet, it may recover its size within 9 months or even outgrow the original size by 50%. The rebound effect is known as thymic rebound hyperplasia, which is often a source of parental concern and a reason for pediatric hospitalization. A true hyperplasia in turn, although rare, may be observed in patients with global disorders, such as untreated hyperthyroidism, sarcoidosis or red cell aplasia [16].
The presented normograms depict a healthy fetal population. In the case of fetuses with cardiac or other defects, their thymus is smaller [19]. Researchers from Gothenburg report that infections and autoimmune diseases are common among patients who during their childhood underwent heart defect correcting surgery and had their thymus resected to gain access to the heart and the great vessels [20]. Also, some primary immunodeficiencies are directly connected with the thymus [19]. Yet, no method has been developed to identify children at risk of developing an autoimmune disease or an immune deficiency [21]. Annually, dozens of suspected primary immunodeficiency cases should be expected, which could be pre-diagnosed thanks to the assessment of the thymus in fetal life, especially in children with suspected heart disease characteristic for DiGeorge syndrome (Fallot tetralogy 20–45%, pulmonary atresia and ventricular septal defect 10–25%, interrupted aortic arch 5–20%, truncus arteriosus 5–10%, ventricular septal defects (conoventricular) 10–50%, isolated aortic arch anomalies 10%) [22]. It is estimated that primary immune deficiency incidence is 1 : 10 000 births [23]. According to the Central Statistical Office of Poland, there were nearly 403 thousand births last year [24], which means that about 43 of them have a primary immunodeficiency. The prevalence of primary immunodeficiency in the Polish population is estimated at over 20 000 [25].
As mentioned before, thymic size can be indicative of thymic function. Diemert et al. revealed a negative correlation between thymic enlargement and the frequency of regulatory T cells in umbilical cord blood, which indicates an association of the developing fetus and the thymus with the neonate’s immune status [26]. The most important matter linked to the development of thymic nomograms in healthy children is the possibility of their use in detecting primary immunodeficiencies, which are accompanied by abnormalities of the size of this organ, or its absence. The fetal thymus ultrasound may be a screening test in the direction of deletion 22q11.2 (DiGeorge syndrome), especially in children who have an inborn heart defect in the echo test [27, 28]. Consequently, the introduction of fetal thymus nomograms into routine USG examinations could serve the purpose of monitoring various disorders linked with intrauterine infections, IUGR or premature labor. Fetuses with a heart defect have an absolute indication for thymic size monitoring as they have an increased risk of perinatal infection, which could be detected before clinical signs develop. Particular attention should also be paid to this group of fetuses in which, in several ultrasound measurements, the size of the thymus does not fall within the normograms for a given gestational age. At this time, an immunological perinatal consultation should be planned in order to qualify for a further possible postnatal vaccination and diagnosis. Information about hypoplasia of the thymus should be noted in the child’s medical history and health book [29].
In conclusion, the coverage of healthy thymus nomograms in the fetal population may be the basis for the identification of fetuses at risk of hypoplasia or thymic hyperplasia, which seems particularly important from the point of view of the detection of potential inborn immunological disorders.