According to World Health Organization data, globally there are around a billion cases of seasonal influenza annually and the infection rate in healthy children is estimated at 10–30% [1, 2].
Seasonal influenza and epidemic are most often caused by type A virus (H1N1 and H3N2 subtypes).
The clinical presentation of influenza characteristically begins with high fever, headache, myalgia, malaise, which can be then followed by cough, pharyngitis, rhinitis and gastrointestinal symptoms. Complications of influenza mostly include pneumonia, acute otitis media, neurological complications, myocarditis and exacerbation of the existing chronic diseases [3].
Oseltamivir is the first-choice antiviral medication for patients and the best results are observed when the child is treated within 48 h of symptom onset [4].
The American Academy of Pediatrics and the Polish Society of Pediatrics recommend yearly influenza vaccination for everyone aged 6 months and older. Moreover, vaccination is particularly recommended for children with risk factors for severe disease [5, 6].
The study aimed to analyze the clinical manifestation and risk factors for influenza in the 2023/2024 epidemic season in a group of hospitalized children.
Methods
The electronic database was searched for patients between the ages of 0 and 18 years diagnosed with influenza who had been admitted to the Department of Infectious Diseases and Pediatrics between 1 December 2023 and 31 March 2024.
Caretakers of children, and patients over 16 years of age signed the appropriate written consent for participation in the observational study.
Influenza virus infections were confirmed in outpatients by various types of rapid antigen tests (RATs). In the remaining patients similar RATs (Assure Tech./Hangzhou/China, Flu A/Flu B/RSV/ADV/MP/COVID-19) or molecular assay reverse-transcription polymerase chain reaction (RT-PCR) on the first day on admission to the hospital were performed.
Patients were excluded if any co-infection with other viruses was diagnosed.
Statistical analysis
The obtained research results were subjected to statistical analysis. The relationship between qualitative variables was assessed using the χ2 test. The normality of the distribution of variables in the study groups was checked using the Shapiro-Wilk normality test. The Mann-Whitney test was used to examine differences between groups. The Pearson correlation coefficient (r) was also used to check the relationship between some variables. The significance level of p < 0.05 was adopted, indicating the existence of statistically significant differences or relationships. The database search and statistical tests were carried out using Statistica 9.1 computer software (StatSoft, Poland).
Results
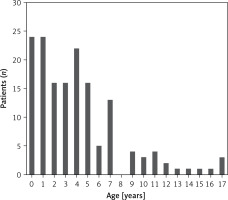
A total of 170 cases of children with influenza were hospitalized in the Department of Infectious Diseases and Pediatrics, 14 were excluded, and 156 were analyzed. Among them 56% were male (88 patients). Median age was 4 years and 4 months (IQR: 3 weeks–17 years and 6 months) (Figure 1). The mean time between symptom onset and hospital admission was 3.3 (IQR: 1–20) days; 72 (46%) patients were diagnosed on an outpatient basis, and 84 (54%) in a hospital emergency department/admission room. Influenza was confirmed by various types of RATs or a molecular assay RT-PCR (150 vs. 6; 96% vs. 4%). Median length of hospitalization was 3.5 (IQR: 1–14) days. There was no correlation between age and the duration of hospitalization (p = 0.864). No admission to the intensive care unit was reported.
The number of admissions reached a peak in February – 58 (37%) patients and there was a predominance of influenza virus type A (87.2%).
Among all children, 40 (25.6%) were registered with one or more risk conditions for severe influenza. The most common were: respiratory diseases – asthma and cystic fibrosis – 12 (30%), then immunological disorders/immunosuppressive treatment – 8 (20%), heart defects – 7 (17.5%) and others (including neurological diseases) – 13 (32.5%). There was no correlation between the presence of a chronic disease and the duration of hospitalization (p = 0.849).
The most reported clinical symptoms were: fever > 38.5ºC – 113 (72%), cough – 140 (90%), vomiting and diarrhea – 66 (42%) and 30 (19%), respectively, dehydration – 118 (76%), hepatitis – 36 (23%), and myositis – 26 (17%). 117 patients required intravenous rehydration (75%).
Complications were observed in 50 (32%) patients. The most common were: pneumonia – 34 (68%), including bacterial and viral – 21 (61.7%) and 13 (38.2%), respectively, then acute otitis media (AOM) – 11 (22%), streptococcal pharyngitis – 4 (8%) and sinusitis – 3 (6%). There was no correlation reported between patients age and the frequency of complications (p = 0.582). Among children with comorbidities, influenza complications were recorded in 17 (42.5%) with pneumonia predominance cases – 12 (70%). There was no statistically significant difference between the presence of chronic disease and the occurrence of complications (p = 0.101), but the incidence of complications significantly prolonged hospitalization time (median 2.9 days vs. 5.1 days; p < 0.001).
100% of patients received antiviral treatment with oseltamivir, 30 (19%) on an outpatient basis, and 126 (81%) on the first day of hospitalization. The mean time between symptom onset and initiation of antiviral treatment was 3.1 days, 82 (52.5%) of analyzed children received treatment within 48 h after symptom onset. In the group of patients in whom oseltamivir treatment was started < 48 h from the onset of symptoms, 19 (23%) had complications, while in the group in which treatment was started > 48 h, complications were diagnosed in 31 (42%) of patients.
Antibiotic therapy was administered to 50 children. It involved most commonly: amoxicillin – 22 (44%), ceftriaxone – 12 (24%) and clarithromycin – 7 (14%).
Discussion
According to the Centers for Disease Control and Prevention (CDC) surveillance reports summarizing the 2023/2024 influenza epidemic season there were 400,000 hospitalizations for influenza so far this season. Approximately annually 10% of infections occur in children younger than 5 years [7].
In our study, the largest group included children up to 2 years of age, which was 30% of all hospitalized patients.
According to Hauge et al., among children hospitalized for influenza, 24.6–26.2% were categorized as having at least one risk condition. Jane et al. indicated that 16.8% of children admitted to the hospital had chronic disease [8, 9]. In our study, similarly to Hauge et al., 25.6% of children were registered with one or more risk conditions. Hauge et al. showed that the most prevalent comorbidities were lung disease (4.9–6.3%), neurological diseases (5.3–7.5%), heart conditions (2.1%) and patients diagnosed as immunocompromised (4.2–5.5%). According to Jane et al., the most prevalent risk conditions include asthma (28.6%), cardiovascular disease (26.5%) and immunosuppressive diseases (20.4%) [8, 9]. In our cohort the most common risk factors were respiratory diseases – 12 (30%).
The frequency of signs and symptoms of influenza in our study supports the results of previous works [10, 11].
Explanation for the high proportion of hospitalized children with no pre-existing risk condition could be that there is a low threshold for hospitalization for the youngest children in Poland. Dehydration is one of the main complications of influenza infection and the children are more difficult to rehydrate and observe at home. In our study 116 (74%) patients without comorbidities were admitted to the hospital, 83 (72%) were dehydrated and all of them required intravenous rehydration.
According to Wrotek et al., the most common influenza complications among all hospitalized patients were pneumonia (31%) and bronchiolitis (23%) [12]. In our study the most common were pneumonia (22%) and AOM (8%). Schober et al. indicated that 71.6% received any antibiotics during their hospitalization [13]. In our study antibiotic therapy was administered to 50 (32%) children. The most commonly administered antibiotic was amoxicillin.
Previous studies have shown that oseltamivir treatment started within 48 h of illness onset, can decrease the risk of intensive care unit admission and mortality. Jane et al. indicated that the mean time between symptom onset and initiation of antiviral treatment was 5.2 ±4.2 days. 28.7% of patients received it within 48 h after symptom onset [9]. In our study the mean time between symptom onset and initiation of antiviral treatment was much shorter – 3.1 days, and much more – 52.5% of patients – received treatment within 48 h. Moreover, treatment initiated > 48 h after the onset of symptoms increased significantly the risk of complications (p = 0.002).
Flu vaccine effectiveness estimates from the CDC show that vaccination 2023/2024 season reduced the risk of flu-related hospitalization by about half for vaccinated children [13]. Unfortunately, despite widespread public funding of influenza vaccines for children in Poland, coverage of vaccination for this population remains constantly low: 2 percentage points (0–4 years old) and 1.9 percentage points (5–14 years old) [14, 15]. In our group, vaccination was recorded in 1 patient.
Our study has several limitations. It was a single-center retrospective study. The diagnosis of influenza infection was made mostly by antigen tests.
In conclusion, the main indication for hospitalization of pediatric patients with influenza in the 2023/2024 epidemic season in Poland was the need for intravenous rehydration. The main risk factors for hospitalization were age under 5 years, comorbidities and the lack of vaccination. There was no correlation between the presence of chronic disease and occurrence of complications, but the incidence of complications prolonged significantly the hospitalization time. The lack of vaccination against influenza in the Polish pediatric population is a significant problem of public health.