Endometriosis is a disease caused by the presence of stromal cells and mucosal lining, similar histologically and hormonally to the eutopic endometrium, outside the uterine cavity. One of the forms of the disease, that can lead to infertility and persistent pelvic pain is an ovarian cyst called endometrioma. Despite numerous studies on endometriosis, the cause of this phenomenon is still not established. Scarce research has been done on the hormonal molecules involved in one of the essentials of endometriosis, which is the hyperestrogenism [1–3]. Falconer et al., in their study, found that overexpression of estrogen receptors enhances the pro-inflammatory response [4]. The estrogen overproduction is also dependent on the other processes like FSHR expression, or FSHR-dependent mechanisms. The follicle stimulating hormone receptor (FSHR) is a receptor of the G protein superfamily located on chromosome 2 [5]. It is a polymorphic receptor affecting many pathways in the female reproductive system such as steroidogenesis and folliculogenesis [6, 7]. Despite the clear link between folliculotropin and estrogenization, there are only a few reports published on the role of FSHR in endometriosis [8, 9].
In endometriosis, the pain symptom has complex nature (receptor pain, neuropathic pain), dominantly caused by inflammation. NGF is a neutropin mainly responsible for the production of new nerve cells and the inhibition of neuronal apoptosis [10]. The role of neutropins in the reproductive system is not entirely clear, but NGF expression is detectable in the reproductive organ and pregnancy [11]. Additionally, data regarding its connection with pain in different forms of endometriosis are scarce.
To sum up, the essence of endometriosis is hyperestrogenism, low progesterone, neurovascularization, and chronic inflammation. The above mechanisms overlap, creating a vicious circle process. In this study, we focused on the expression of FSHR and NGF in endometriomas since there is a gap of knowledge regarding its influence on cyst formation and pain pathways in the course of cyst enlargement. This study was conducted using a tissue microarray (TMA) to improve quality and accuracy for further immunohistochemical (IHC) analyses [12].
Methods
Patients selection
A total of 101 patients were included in the study, operated on due to ultrasound diagnosis of benign ovarian cysts and accompanying symptoms, within the years 2018–2020. Patients of childbearing age at the time of surgery and patients in the first phase of their menstrual cycle were randomly selected for the study after signing informed consent. Those who did not meet the inclusion criteria (benign ovarian cyst, age 18–50, the first phase of the menstrual cycle, informed consent signed), met the exclusion criteria or had incomplete data were excluded from the study. A qualification for laparoscopy was beyond the study assessment. Exclusion criteria were as follows: patients in the second phase of the menstrual cycle according to menstrual dating (≥ 14 days of the cycle), patients on hormonal therapy during hospital stay, patients after hysterectomy, and patients receiving chemotherapy or other systemic treatment. Also patients with suspicious or malignant lesions according to intraoperative pathology reports were excluded from the study.
The detailed group distribution was as follows: the study group (SG) comprised 45 patients with endometrioma, whereas the control group (CG) comprised 56 patients diagnosed with a different type of the ovarian cyst (dermoid, serous, follicular, hemorrhagic, simple, mucinous cyst). Among the examined women, 61 patients were diagnosed intraoperatively with any form of endometriosis: endometrioma (endometrial ovarian cysts – EOC), or endometrioma and peritoneal endometriosis (PE) (EOC + PE) or diagnosed with peritoneal endometriosis and accompanied ovarian cyst of other origin (other ovarain cyst – OOC) (OOC + PE). In 40 patients, endometriosis was not diagnosed.
Summing up, we created two divisions for further analysis. The first was made up of patients primarily qualified as SG (endometrioma) and CG (non-endometrioma). The other consisted of patients with diagnosis of any type of endometriosis: EOC, EOC + PE, and OOC + PE related to patients with OOC without diagnosis of endometriosis (OOC).
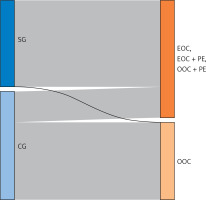
The distribution of patients is presented in Figure 1.
Figure 1
Sankey diagram – the distribution of endometriosis in the study group (SG = 45), the control group (CG = 56). Endometriosis = EOC + (EOC + PE) + (OOC + PE)
The postoperative, second histopathological examination consisted of routine HE stainings to verify the initial diagnosis, evaluate the representativeness of the sample, as well as to assess the possibility of TMA preparation. A TMA performance and IHC reactions were conducted at the Department of Histology and Embryology of the Wroclaw Medical University as described below. The study was approved by the Bioethics Committee of the Medical University of Lodz (RNN/168/18/KE).
Tissue microarray method (TMA) and immunochemistry (IHC)
A hematoxylin-and-eosin-stained (HE) 6 μm thick paraffin sections were prepared to verify the histopathological diagnosis and assess the suitability of the samples for further analysis. All further steps were performed as described by Ciesielska et al. [12]. Available slides were digitized by the histological scanner Pannoramic MIDI (3DHistech, Sysmex Suisse AG, Horgen, Switzerland). Then, for TMA construction, from the corresponding paraffin donor blocks, triplicate tissue core punches (2 mm) for every case were obtained (TMA Grand Master; 3DHistech). Immunochemistry was performed on 4 μm paraffin sections from the TMA blocks.
Evaluation of the IHC reactions
The expression of both tested proteins was cytoplasmic and was assessed with the usage of Pannoramic Viewer Digital image. The analysis was carried out by two independent researchers using immunoreactive scale (IRS) by Remmele and Stegner [13]. This scale uses the percentage of positively stained cells (A) and the staining intensity of the reaction (B). The final result is the product of these two parameters (A × B).
Statistical analysis
Statistical analysis was performed using RStudio 4.2.0. P-values < 0.05 were considered statistically significant. The Shapiro-Wilk test was used to check results for normal distribution. None of the investigated variables showed normal distribution. The Mann-Whitney-Wilcoxon test and Kruskal-Wallis tests were used to investigate differences between groups of continuous variables. To compare qualitative features, χ2 test was used whereas to compare linear relations, Pearson correlation coefficient was used.
Results
The characteristics of the groups are presented in Table I.
Table I
Characteristics of the studied population. SG = study group = endometrioma (n = 45), CG = control group = other ovarian cyst, n = 61; p-value < 0.05 considered as statistically significant
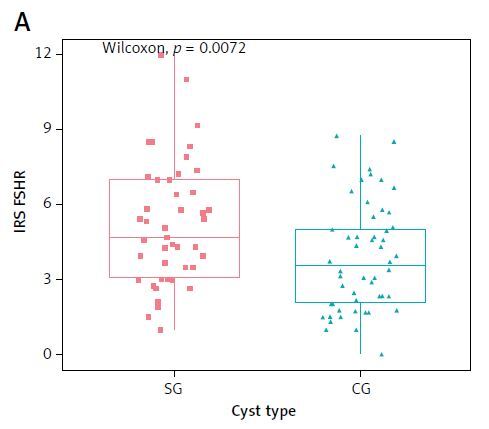
The study presented a stronger FSHR expression in SG vs. CG (p = 0.0072) and simultaneously in subgroups EOC + PE vs. OOC (p = 0.011) – Figure 2.
Figure 2
The immunohistochemical expression of the follicle-stimulating hormone receptor (FSHR) in main groups SG and CG (A) as well as of the nerve growth factor (NGF) in SG and CG (B). C – expression of the follicle-stimulating hormone receptor (FSHR) in subgroups: endometriosis vs. OOC; D – expression of NGF in subgroups: endometriosis vs. OOC
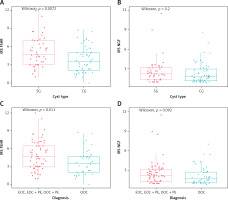
Since some patients from the control group were also diagnosed with peritoneal endometriosis (intraoperative diagnosis), we decided to differentiate patients into four subgroups to assess them in detail. All four groups were then compared and a statistically significant difference was observed (p = 0.046). A post-hoc analysis showed a significantly stronger FSHR expression in SG vs. CG (p = 0.049) and in EOC + PE vs. OOC (p = 0.011). No significant differences were observed among the remaining groups.
The NGF expression analysis showed no significant differences in the studied groups: SG vs. CG (p = 0.2), EOC + PE vs. OOC (p = 0.092) – Figure 2. Subgroups were compared as in the case of FSHR and no significant differences were observed in NGF expression (p = 0.32), with the tendency near/close to significance between EOC + PE vs. OOC (p = 0.092).
As suspected, painful menstruation was reported more often by patients with diagnosed EOC (62.22%, n = 28, p = 0.0002) and EOC + PE (57.38%, n = 35, p < 0.0001). Pain was the only factor that significantly differentiated groups (Table I).
Relations between FSHR and NGF expression and the cyst diameter were also analyzed with no statistically significant correlation observed. The study included also multiple additional variables, such as age, BMI, menstruation history (menarche, cycle, length), and conception history (pregnancies, miscarriages, delivery type, infertility). Correlation was used to compare the studied variables, yet no statistically significant correlations were observed between these variables and the sizes of cysts nor FSHR and NGF expressions.
Discussion
Previous studies on FSHR proved that endometrial foci behave like ovarian tissue, there is an isolated overproduction of estrogen, folliculotropin and aromatase, which secondarily increases the local production of the above-mentioned hormones [8–10]. To the authors’ knowledge, this study is among the first ones that evaluate FSHR and NGF expression in ovarian cysts of varied origins by TMA technique. Even though ovarian cysts are quite common during reproductive age, with the predominant occurrence of endometrial findings in the late reproductive period, there are only scarce data on the differences in their molecular print [14]. This knowledge enables an understanding of pain pathogenesis and endocrine disorders in endometriosis which can directly help individualize the therapy. In our study a higher FSHR expression was observed in the cyst wall of endometriosis patients’ group vs. control group what confirms its role in local endocrine regulation. Robin et al. were one of the first researchers that described the role of FSHR in endometriosis, but not in endometriomas [8]. Another study involving FSHR was carried out by Ponikwicka-Tyszko et al. [9]. They confirmed the FSHR role in hyperestrogenism in deep endometrial foci where its increased expression elevated aromatase activity, thus estrogen production. As in the case of Robin et al., their study confirmed FSHR expression in healthy patients’ endometrium and proved expression differentiation depending on the menstrual cycle phases. Based on this knowledge, we chose only patients in the first phase of the cycle to our study. The NGF and FSHR correlation described in the literature and the vicious circle phenomenon, e.g. FSHR overexpression results in hyperestrogenism, constitute an important finding [15, 16]. Hyperestrogenism and active inflammation intensify NGF expression. Neutropin overexpression increases the creation of new foci and new neural and vascular interactions among the foci. The role of NGF has already been widely studied in endometriosis pathogenesis in multiple contexts, yet the authors were the first ones to compare its expression in various types of ovarian cysts.
The TMA method used in the study contributed to the elimination of random tissue selection without endometriosis foci since they could bring nonobjective data. An indisputable advantage of the technique is the possibility to choose a small tissue part, which in the case of small lesions is important, and also protects the preparation against damage. Moreover, the method allowed for simultaneous study of specimens’ expression from many patients, thus standarizing the reaction environment [12, 13]. However, results obtained in the TMA technique should be also confirmed by molecular methods and the lack of them can be considered as a major limitation of the study.
In conclusion, even though FSHR expression in endometrioma is not correlated with endometriosis symptoms, its role in the formation of the cysts is undisputed. Since antibodies against FSHR are already used in in vitro studies in ovarian cancer, maybe targeting this receptor in endometriomas could also provide a new way for conservative management of endometriosis [17, 18]. Undoubtedly, further investigations are necessary to create tailored, effective, and affordable therapies for endometriosis as well as for endometriosis-associated infertility.