Introduction
Upper tract urothelial carcinoma (UTUC) is an uncommon disease that accounts for only 5% of all urothelial tumours and 10% of all renal tumours [1, 2]. It can extend from the pyelocaliceal cavities to the ureteral orifice. Unifocal, small, low-grade tumours with papillary growth and no invasive potential can be treated with kidney-sparing surgery; however, approximately 60% of patients present with an invasive disease [3]. The gold standard of treatment for patients with an invasive disease is radical nephroureterectomy (RNU) with excision of the bladder cuff. Radical treatment should also be considered in all patients with a normal contralateral kidney when one of the following factors occurs: hydronephrosis, tumour size > 1 cm, high-grade tumour on biopsy, multifocal disease, or previous radical cystectomy for bladder cancer [4].
Clear knowledge on prognostic factors is necessary to identify patients with adverse cancer-related features who may in turn benefit from more aggressive and/or additional treatments. The influence of tumour stage, tumour grade, and lymph node status on prognosis has been well established [4–6]. Another potential prognostic variable is the tumour location. According to the guidelines of the European Association of Urology, a tumour within the ureter is a factor of poor prognosis for intravesical recurrence; however, information on its influence on survival after RNU is limited compared with that of other prognostic factors [4]. Most studies that reported tumour location as a significant factor for cancer-specific survival (CSS) or overall survival (OS) were limited by their small sample size, case selection bias, single-centre nature, or a heterogeneous population [7, 8]. Furthermore, the definition of ureteral involvement (UI) differs across studies. Consequently, this generates bias in evaluating the association between survival and the presence of tumour within the ureter. Therefore, the goal of this study was to perform a robust evaluation of the prognostic value of UI in UTUC by conducting a systematic review of the literature and a meta-analysis of available data.
Material and methods
Search strategy
Two authors (K.K. and A.L.) independently performed an electronic bibliographic search of the Medline, Scopus, and Web of Knowledge databases. The following search keywords were used: “cancer specific survival” or “overall survival” or “survival” and “radical nephroureterectomy” or “upper urinary tract carcinoma” or “transitional cell carcinoma” or “urothelial carcinoma” and “tumor location” or “ureteral involvement” or “prognosis” or “risk factors”. Abstract books of major international meetings (European Association of Urology, American Urological Association, European Society of Medical Oncology, and American Society of Clinical Oncology) were hand-searched for potentially relevant studies. Moreover, the references of included studies, and those of a previous systematic review, were checked [9, 10]. All included studies were published in English. These searches were performed without time restriction. The last search was run on 20 March 2018.
Inclusion criteria
Based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, the Population, Intervention, Comparator, Outcome, and Study (PICOS) design approach was used to describe study eligibility [11]. Studies were considered relevant to this meta-analysis if they compared patients diagnosed as having UTUC with UI (P) who underwent RNU (I) with those with carcinoma localised only in the renal pelvis and calyces (C) to determine the role of UI as a predictor of CSS or OS (O) using multivariable Cox proportional hazards regression analysis (S). Meeting abstracts, case reports, editorials, commentaries, letters, and review articles were excluded.
The following criteria were used to select the studies comparing UI with pelvicalyceal tumours:
large studies that included more than 100 patients;
studies that provided a definition of UI;
studies that excluded patients who underwent contemporary radical cystectomy because of a concomitant muscle-invasive bladder cancer or with history of muscle-invasive bladder cancer;
studies that provided hazard ratios (HRs) from multivariable Cox proportional hazards regression analysis with their corresponding 95% confidence intervals (CIs) or studies that provided enough data to calculate CIs.
When more than one study reported results from the same patient cohort, we selected one study with the largest sample size to avoid duplication.
Systematic review process
After removal of duplications, titles and abstracts of 1250 studies were screened by two authors (K.K. and A.L.) for initial study inclusion [12]. Eighty-five potentially relevant studies were assessed for eligibility based on a full-text evaluation, and 14 studies that met all the inclusion criteria were included in the meta-analysis. The studies included in the analysis were published in 2009–2016. Any disagreements between the two reviewers were resolved by discussion; further disagreements were resolved by consensus with the senior author (M.S.). All authors agreed on the final list of included articles. Moreover, we attempted to contact three authors for additional information; two authors responded by providing additional data [8, 9]. A PRISMA flow diagram of the study selection is shown in Figure 1.
Quality of data assessment
All included articles were observational studies and/or retrospective series. The Newcastle-Ottawa Scale, which is an instrument recommended by the Cochrane Collaboration to evaluate the design of nonrandomised studies, was used to assess the quality of the studies. Each study was assessed using the star system, which included three perspectives: selection of the study groups, comparability of the groups, and evaluation of the outcome of interest. Two reviewers (K.K. and A.L.) independently performed the assessment. Quality scores for all studies ranged from 7 to 8 stars (Table I). The trials with seven or more stars were considered to be of sufficient quality for meta-analysis.
Table I
Baseline characteristics and quality assessments of included studies
| Study | Country | Recruitment period [years] | No. of patients | Age [years] | Gender (female/male) | Definition of ureter involvement | NCT (%) | Follow-up (months) | NOS | Covariates associated with CSS or OS |
|---|---|---|---|---|---|---|---|---|---|---|
| Isbarn et al. (2009) [13] | USA | 1988–2004 | 2824 | RP: Md 72 (R 27–99) U: Md 73 (R 29–96) | 1158/1666 | Only ureter plus dominant lesion | NA | RP: Md 45 (R 1–203) U: Md 40 (R 1–203) | 8 | Age, pT stage, LN, tumour grade |
| Raman et al. (2010) [15] | Globe | 1987–2007 | 1249 | Md 70 (R 27–97) | 403/846 | Only ureter plus dominant lesion | NA | Md 49 (R 0.1–250) | 8 | pT stage, LN, tumour grade |
| Favaretto et al. (2010) [16] | USA | 1995–2008 | 249 | Md 72 (IQR 64–77) | NA | Only ureter plus dominant lesion | 0 | Md 48 (IQR 23–92) | 8 | pT stage, LN |
| Yafi et al. (2011) [17] | Globe | 1990–2010 | 637 | Md 68 (IQR 61–75) | 207/430 | 1. Only ureter 2. Both locations | 0 | Md 37 (IQR 18–68) | 8 | pT stage, tumour grade, concomitant CIS, LVI |
| Milojevic et al. (2011) [18] | Serbia | 1999–2009 | 133 | RP: Mn 66.7 (SD 8.9) U: Mn 66.6 (SD 8.9) | 56/78 | Only ureter plus dominant lesion | 0 | Md 35 (R 2–113) | 7 | BEN area, previous carcinoma not invading bladder muscle, tumour focality, tumour grade, pT stage, LVI |
| Ouzzane et al. (2011) [19] | France | 1995–2010 | 609 | Md 70 (R 62–76) | 194/415 | 1. Only ureter 2. Both locations | 0 0 | Md 29 (IQR 12–54) | 8 | Age, pT stage, LN, LVI, tumour grade |
| Chromecki et al. (2012) [20] | Globe | 1987–2007 | 2510 | Md 69.2 (IQR 54.1–84.2) | 811/1681 | Only ureter | 0 | Md 45 (IQR 0–106) | 8 | Age, pT stage, tumour architecture, LVI, adjuvant chemotherapy |
| Mouracade et al. (2012) [14] | France | 1985–2005 | 269 | Mn 67 (SD 7) | 73/196 | Only ureter plus dominant lesion | NA | Mn 80.6 | 8 | Period of diagnosis |
| Zhang et al. (2013) [21] | China | 2000–2010 | 217 | Md 69 (R 62–81) | 87/130 | Only ureter | 0 | Md 52 (IQR 12–78) | 8 | Tumour stage, preoperative hydronephrosis |
| Rouprêt et al. (2013) [22] | Globe | 1987–2010 | 3387 | Md 69 (IQR 60.5–82.3) | 1106/2281 | 1. Only ureter 2. Both locations | 0 | Md 43 (IQR 18–81) | 7 | Age, pT stage, LN, LVI, tumour grade |
| Waseda et al. (2015) [23] | Japan | 1995–2013 | 1068 | Md 70 (IQR 62–76) | 310/758 | Only ureter plus both locations | 0 | Md 40 (IQR 17–77) | 8 | Age, pT stage, LN, LVI |
| Kim et al. (2015) [24] | Korea | 1997–2012 | 445 | Md 64.8 (IQR 57.5–71.3) | 103/342 | 1. Only ureter 2. Both locations | 0 | Md 50 (IQR 25–102) | 8 | Age, pT stage, tumour grade, LVI, SM |
| Tai et al. (2016) [25] | Taiwan | 1996–2009 | 503 | Md 68 (IQR 60–75) | 254/249 | Only ureter plus dominant lesion | 0 | Md 52 (IQR 23–77) | 8 | Age, pT stage |
| Shibing et al. (2016) [26] | China | 2002–2012 | 795 | NA | 333/462 | 1. Only ureter 2. Both locations | 0 | Md 32 (IQR 17–60) | 7 | pT stage, tumour grade, LN, LVI, CVH, tumour architecture, tumour size, SM |
[i] CSS – cancer-specific survival, CT – chemotherapy, CVH – concomitant variant histology, IQR – interquartile range, LN – lymph node status, LVI – lymphovascular invasion, Md – median, Me – mean, NA – not available, NCT – neoadjuvant chemotherapy, NOS – Newcastle-Ottawa Scale, OS – overall survival, pT stage – pathologic tumour stage, R – range, RP – renal pelvis, SD – standard deviation, SM – surgical margin status, U – ureter.
Data extraction
The following information was extracted from each study: study design, name(s) of author(s), year of publication, recruitment period, number of patients, age, gender, definition of UI, neoadjuvant chemotherapy, and median follow-up period. Subsequently, HRs for UI as a potential predictor of CSS and OS were also obtained. To perform a cumulative analysis, HRs from multivariable Cox proportional hazard regressions analysis with their corresponding 95% CIs were extracted. Two reviewers (K.K. and A.L.) independently performed the data extraction. The other authors (A.G. and M.S.) verified the data.
Outcome measures
Pooled HRs were used to assess the prognostic role of UI in UTUC. Hazard ratios for CSS and OS were calculated. Patients with and without UI were compared. The outcomes of individual studies were evaluated based on the definition of UI. The following four subgroups were identified:
tumour present only in the ureter;
tumour present only in ureter or tumour in the ureter as a dominant lesion in the case of multifocal tumours;
tumours within two distinct areas (renal pelvis and ureter);
tumour present only in the ureter and tumours within two distinct areas.
Furthermore, the pooled relative risk (RR) for locally advanced stage UTUC (≥ pT3) located within the renal pelvis and the ureter was estimated.
Statistical analysis
The I2 statistic was employed to detect heterogeneity across the different studies. The I2 statistic describes the percentage of the variability in effect estimates, and a value > 50% indicated heterogeneity. If no evidence of heterogeneity was found, the fixed-effect inverse variance-weighted method was used to pool the effect size; otherwise, a random effect model was used. Publication bias evaluation was performed with a visual inspection of a funnel plot and using a trim and fill method. Statistical analysis was performed using Review Manager version 5.3 (Cochrane Collaboration, Copenhagen, Denmark) and Comprehensive Meta Analysis version 3 (Biostat, New York, USA).
Results
The 14 studies in this meta-analysis included 14,895 participants [13–26]. The baseline characteristics of the included studies are summarised in Table I. The number of patients in each selected study ranged from 133 to 3387 (mean 1064, median 623). The female to male ratio was approximately 1 : 2. Eight studies analysed UI as a tumour present only in the ureter. In six of those studies UI was additionally defined as a dominant tumour within the ureter, in the case of multifocal tumours. A dominant lesion was clarified as that with the highest pathologic tumour stage. Five studies analysed two definitions of UI separately: as a tumour located only in the ureter (1) and as a tumour located in two distinct locations (ureter and renal pelvis) (2). Only one included study assessed together a tumour located only in the ureter and tumours in the two distinct locations as UI. The included studies were performed in different geographical areas: four studies were global trials, three studies were conducted in Europe, two studies were conducted in North America, and five studies were conducted in Asia.
Cancer-specific survival after RNU in patients with UI were compared in all studies. A meta-analysis of these studies indicated statistically significant differences in CSS between both groups (HR = 1.52, 95% CI: 1.31–1.76; p < 0.001; Figure 2). The analysis showed that UI worsened CSS. Moreover, significant heterogeneity was observed across studies (I2 = 64%). The random effects model was used, and funnel plot inspection revealed presence of publication bias. The trim and fill method suggested that three studies were missing and showed that the HR was 1.39 (95% CI: 1.18–1.62).
Figure 2
Meta-analysis of the effect of ureteral involvement on cancer-specific survival in multivariable analysis
A subgroup analysis showed no difference in CSS when UI was defined as a tumour located only in the ureter or as a dominant lesion in the ureter (subgroup 2; HR = 1.11, 95% CI: 0.93–1.32; p < 0.26). Heterogeneity was not observed (I2 = 0%). Analysis with other subgroups emphasised better CSS in patients without UI. Sensitivity analysis by sequential omission of individual studies was performed to assess the stability of the results in subgroups with significant heterogeneity. The analysis indicated that the studies of Chromecki et al. [20] and Rouprêt et al. [22] were the main sources of heterogeneity in the first and third subgroups, respectively.
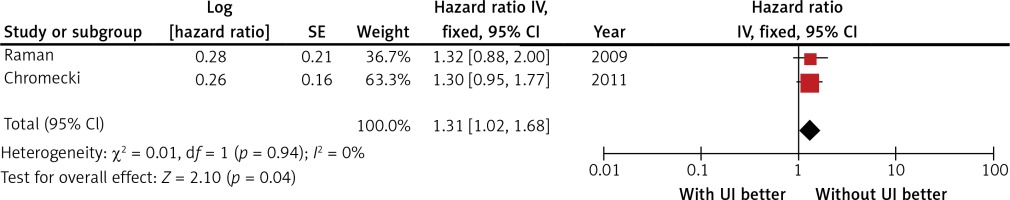
Two studies compared HRs for CSS among patients with organ-confined disease. The comparison included 2353 patients. The meta-analysis of those studies also emphasised differences in CSS. Among patients with organ-confined diseases, those with UI had a worse prognosis (HR = 1.31, 95% CI: 1.02–1.69; p = 0.035; Figure 3). Heterogeneity was not observed (I2 = 0%), and the fixed effects model was used.
Figure 3
Meta-analysis of the effect of ureteral involvement on cancer-specific survival in patients with organ-confined upper tract urothelial carcinoma
Ureteral involvement as a predictor of OS was reported in five studies. The data were not homogeneous (I2 = 78%), and the random effects model was used. Cumulative analysis showed that UI is also a significant predictor of OS. The pooled HR for patients with versus those without UI was 1.39 (95% CI: 1.11–1.74; p = 0.004). Funnel plot inspection revealed no indication of publication bias. Multivariable-adjusted HRs for each study are shown in Figure 4.
Figure 4
Meta-analysis of the effect of ureteral involvement on overall survival in multivariable analysis
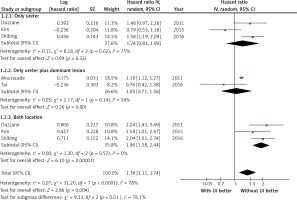
Furthermore, a subgroup analysis indicated that a difference in OS was only observed in the third subgroup (renal pelvis vs. renal pelvis and ureter). The pooled HR was 1.96 (95% CI: 1.58–2.44). Heterogeneity was not observed (I2 = 0%). When UI was defined as a tumour located only in the ureter (subgroup 1) or as a tumour located only in the ureter and as a dominant lesion in that location (subgroup 2), no differences in OS were found. Sensitivity analysis indicated that the study of Kim et al. affected the summary result in the first subgroup [24].
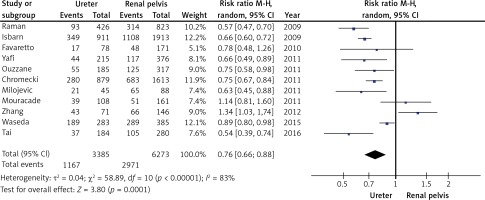
Ten studies provided sufficient information to calculate the RR for locally advanced disease (pT3/4) stratified by tumour location (ureter vs. renal pelvis). In those studies, the number of patients who had organ and non-organ-confined diseases were 6272 and 4478, respectively. Statistically significant differences were found in the meta-analysis of tumour location and tumour stage. Patients with a tumour located in the ureter were found to have a lower risk of non-organ-confined disease (RR = 0.76, 95% CI: 0.66–0.88; p < 0.001; Figure 5). Heterogeneity was significant (I2 = 83%); sensitivity analysis was conducted to find the source of heterogeneity among all the included studies. The results showed that no single study affected the summary of risk estimates, which indicates that our results are reliable. Visual funnel plot inspection suggested no publication bias.
Discussion
Our study is the first meta-analysis that attempts to assess the influence of UI, which has different definitions, on prognosis. Previous meta-analyses did not consider patients with ureteral tumours in subgroups. In several studies, tumours located in both the renal pelvis and ureter were classified as multifocal tumours and were not included in the analysis of UI. However, some studies classified patients with multifocal tumours as unifocal based on the site of the dominant lesion. Consequently, identifying the true value of UI is difficult. The variety of approaches in determining UI limits the use of ureteral tumours as a prognostic factor.
The results of our meta-analysis demonstrated that UI worsens CSS and OS, and analysis of a subset of patients with organ-confined disease revealed that among cases with similar pathologic stages, better CSS was found if the tumour was located in the renal pelvis. Several plausible explanations for the aforementioned results exist. First, the anatomical characteristics of the ureter facilitate the spread of tumour cells. The ureter has a thin layer of surrounding adventitia that contains a plexus of blood and lymphatic vessels. Waseda et al. proved that lymphovascular invasion (LVI) is more often observed in ureteral localisation [23], whereas Lee et al. emphasised that LVI is a practical prognostic parameter, especially in ureteral tumours [27]. Conversely, tumours located in the renal pelvis are surrounded by renal parenchyma and perihilar adipose tissue, which is a natural barrier that is capable of containing the spread of tumour cells, and provides wider surgical margins and enables easier resection. However, some studies showed worsened survival outcomes for pelvis tumours, which could be explained by the thinner muscularis layer in the renal pelvis compared with that in the lower part of the ureter where 70% of ureteral tumours are located. Nevertheless, these studies were restricted by their small sample sizes [28, 29].
Higher hydronephrosis prevalence in ureteral tumours could also explain the differences in survival. Hydronephrosis is a well-established prognostic parameter for bladder cancer, and the recent meta-analysis by Cao et al. indicated that hydronephrosis is correlated with worsened OS among patients with UTUC [30]. In addition, Morizane et al. showed that preoperative serum creatinine level has an effect on CSS among patients who underwent nephroureterectomy [31]. The influence of hydronephrosis on survival could be mainly explained by the associated multiorgan dysfunction. Impaired renal ability to maintain fluid and electrolyte homeostasis results in accumulation of toxic metabolic products, which subsequently increases the risk of cardiovascular events and deaths from any cause independent of comorbidities [32]. Preoperative hydronephrosis in patients with UTUC is also related to more pronounced glomerular filtration rate (GFR) deterioration after RNU [33], thereby seriously restricting the use of cisplatin-based adjuvant chemotherapy [34].
The difference in survival between pelvic and ureteral tumours among patients with organ-confined disease was a surprising finding in this meta-analysis. Some authors previously attempted to assess survival in patients with UTUC based on tumour stage and location. Most of the studies reported similar prognosis when ureteral and renal pelvic tumours were matched for tumour stage [35, 36]. However, Kim et al. predicted the development of muscle-invasive bladder cancer (MIBC) after RNU. Their study emphasised that tumour location within the ureter was an independent risk factor for MIBC and that patients with MIBC had a worsened CSS compared with those without MIBC. Additionally, stratification analysis for matching pathologic stage was performed and revealed that MIBC was correlated with significantly poorer CSS only in patients with stage pTa/T1 UTUC, while no difference in CSS among patients with tumour stage ≥ pT2 was noted [37].
This current analysis also emphasised some interesting differences in pathologic characteristics based on the tumour location. Locally advanced tumours (pT3/4) were more frequently observed in the pyelocaliceal system, which is in contrast to findings from a previous meta-analysis by Wu et al., who reported no difference in the percentage of pT3/4 lesions between the two locations [10]. This conflicting finding could be attributed to differences in exclusion criteria. In the study of Wu et al., the stage distribution of tumours might have been influenced by the patients who received preoperative treatment and the inclusion of studies with a smaller sample size. Symptomatic obstruction of the urinary tract is frequently observed in patients with ureteral tumours, which could explain the earlier diagnosis and the lower tumour stage.
The subgroup analysis according to the definition of UI revealed a different result in one particular group. The estimated pooled HRs indicated that UI worsened CSS and OS. However, in subgroup 2, where UI was defined as the dominant lesion in the ureter, the analysis found no discrepancy in survival between patients with and those without UI. The higher percentage of more advanced tumours in the renal pelvis suggests that patients with both regional tumours were included in the non-UI group. Moreover, Waseda et al. suggested that tumours in both locations should be considered as UI independently of tumour stage in the ureter. The author proved that no difference in survival between patients with both regional tumours and patients with tumours located only in the ureter exists [23]. Therefore, patients with tumours in two distinct locations should be taken into account in the analysis of UI instead of the analysis of tumour focality.
This meta-analysis has some limitations. The included studies were mainly retrospective – hence the heterogeneity in the analysis. Some studies possibly failed to identify survival because of relatively short follow-up periods. The included studies were performed in diverse geographical settings (e.g., Europe, Asia, North America), and some authors presented international and multi-institutional results. This geographical diversity might have generated differences in several factors influencing tumour characteristics, including genetic, cultural, and environmental factors [38]. Additionally, our meta-analysis included five studies from Asia, where the population of patients with UTUC is known to differ from that in Western countries and where UTUC is most common in females [39, 40]; transitional cell carcinoma is the most common urological cancer in some Eastern countries [8]. Furthermore, tumours frequently occur in the ureter, and multifocality is not a predictive factor for prognosis [40]. Most of the included studies excluded patients with neoadjuvant therapy, and patients received adjuvant therapy in some studies. The analysis also failed to consider other factors that could be a source of heterogeneity and reflected in the results, such as surgical technique, tumour size, or presence of carcinoma in situ.
In conclusion, despite the limitations, the current meta-analysis was able to emphasise worse overall and cancer-specific survival of ureteral vs pelvicalyceal tumours. Our results represent the most robust proof of the difference in the biological potential of ureteral UTUC. We also recommend that researchers should not consider the presence of both regional tumours as unifocal based on the dominant lesion.