Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / RESEARCH PAPER
Metformin attenuates the progression of esophageal squamous cell carcinoma by downregulating miR-141-3p to enhance SCEL
1
Thoracic Surgery, Wuhan Third Hospital, China
2
Department of Intensive Care Unit, Wuhan Third Hospital, China
Submission date: 2022-09-19
Final revision date: 2022-11-25
Acceptance date: 2022-12-11
Online publication date: 2023-01-20
KEYWORDS
TOPICS
ABSTRACT
Introduction:
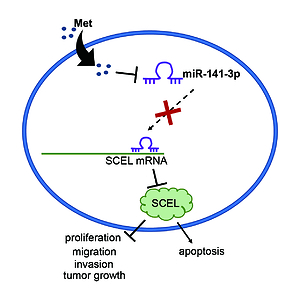
Metformin (Met), a first-line oral anti-type 2 diabetes medication used globally, has been shown to hinder cancer progression via regulation of microRNAs (miRNAs). The previous reports on the relationship between Met use and the risk of esophageal squamous cell carcinoma (ESCC) have been controversial. Hence, this study aimed to explore how Met affected ESCC progression and the underlying molecular mechanism.
Material and methods:
Cell migration, viability and invasiveness were respectively investigated using wound healing, CCK-8 and transwell assay. The expressions of miR-141-3p and Sciellin (SCEL) mRNA were examined by mean of quantitative real-time PCR (qRT-PCR) assay, and SCEL protein level was quantified by western blotting. In vivo tests of Met were performed on animals. The predicted binding of miR-141-3p to SCEL was more evidenced by dual-luciferase assay.
Results:
Met treatment in ESCC cell largely impaired cell viability, migration and invasiveness. MiR-141-3p showed high expression in ESCC and was downregulated in ESCC cells after Met treatment. MiR-141-3p upregulation in Met-administered ESCC cells largely restored cell proliferative ability, migration and invasiveness. MiR-141-3p also attenuated the anti-tumor effect of Met in vivo. MiR-141-3p targeted SCEL whose expression was declined in ESCC, and SCEL expression was reinforced in ESCC cells after Met treatment. MiR-141-3p upregulation depleted SCEL expression and thus partially abolished the anti-cancer impacts of SCEL in Met-treated ESCC cells.
Conclusions:
Met restrains the progression of ESCC by regulating the miR-141-3p/SCEL axis. Our findings clearly show that Met is associated with a lower risk of ESCC, and its anticancer effect could potentially be used to treat ESCC.
Metformin (Met), a first-line oral anti-type 2 diabetes medication used globally, has been shown to hinder cancer progression via regulation of microRNAs (miRNAs). The previous reports on the relationship between Met use and the risk of esophageal squamous cell carcinoma (ESCC) have been controversial. Hence, this study aimed to explore how Met affected ESCC progression and the underlying molecular mechanism.
Material and methods:
Cell migration, viability and invasiveness were respectively investigated using wound healing, CCK-8 and transwell assay. The expressions of miR-141-3p and Sciellin (SCEL) mRNA were examined by mean of quantitative real-time PCR (qRT-PCR) assay, and SCEL protein level was quantified by western blotting. In vivo tests of Met were performed on animals. The predicted binding of miR-141-3p to SCEL was more evidenced by dual-luciferase assay.
Results:
Met treatment in ESCC cell largely impaired cell viability, migration and invasiveness. MiR-141-3p showed high expression in ESCC and was downregulated in ESCC cells after Met treatment. MiR-141-3p upregulation in Met-administered ESCC cells largely restored cell proliferative ability, migration and invasiveness. MiR-141-3p also attenuated the anti-tumor effect of Met in vivo. MiR-141-3p targeted SCEL whose expression was declined in ESCC, and SCEL expression was reinforced in ESCC cells after Met treatment. MiR-141-3p upregulation depleted SCEL expression and thus partially abolished the anti-cancer impacts of SCEL in Met-treated ESCC cells.
Conclusions:
Met restrains the progression of ESCC by regulating the miR-141-3p/SCEL axis. Our findings clearly show that Met is associated with a lower risk of ESCC, and its anticancer effect could potentially be used to treat ESCC.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.