Introduction
During the last decades, a dramatic decline has been observed in patients with acute myocardial infarction (AMI) in hospitalizations, case-fatality rates and long-term survival and events. This reflects the widespread application of evidence-based treatment such as reperfusion therapy during the acute stage as well as immediate and long-term implementation of preventive strategies. However, the recent years of the pandemic have reversed these positive trends and pose new challenges to healthcare providers and physicians.
Guidelines for the treatment of acute coronary syndromes (ACS), either ST-segment elevation myocardial infarction (STEMI) [1] or non-ST-segment elevation myocardial infarction (NSTEMI) [2], and cardiovascular prevention [3] such as those of the European Society of Cardiology (endorsed by the French Society of Cardiology) allow broad access to the different available evidence-based strategies either during hospitalization or after discharge for secondary prevention. However, several registries have demonstrated that, on one hand, the application of these guidelines, especially for further follow-up after discharge, is often not optimal and that, on the other hand, a high residual risk of major events persists, even for “apparently stable” patients in secondary prevention. One explanation could be a lack of adequate communication between cardiologists and other health professionals either directly involved in ambulatory cardiovascular care (including general practitioners, pharmacists, nurses and physiotherapists) or susceptible to delivering care which can interfere with the ongoing cardiovascular treatment (surgeons, anaesthesiologists, dental surgeons). Another important reason is that there are large country-to-country differences in drug availability (including reimbursement criteria for new drugs) and physicians’ and patients’ knowledge.
Thus, transition of care (TOC) from the hospital to an ambulatory setting and further follow-up appears to be a crucial period. Therefore, after an ACS, optimization of the management of coronary outpatients (including considering of so-called coordination care), according to evidence-based guidelines, could benefit from a better consideration of real-life experience. The main goal of this position paper is to provide to French physicians (non-hospital cardiologists and general practitioners), and indirectly to other health professionals, convenient guidance for better application of EBM such as presented in reference guidelines for the management of patients with ACS after discharge from hospital.
Material and methods
A group of French and recognized international experts involved in the initial and follow-up care of patients with ACS was invited by the Collège National des Cardiologues Français and the Collège National des Cardiologues des Hôpitaux to form a working group (Transition Care group). The first objective of the experts was, according to registries of clinical practice and to their own experience, to identify which points of the most recent guidelines on coronary patients [1–3] were most often missed. The second objective was to propose actions and tools aimed at providing a practical guide for optimisation of long-term follow-up of these patients. Even though several treatment strategies are available, this article is focused on patients who have undergone percutaneous coronary intervention (PCI).
Our findings are arranged by topic, beginning when the patient is discharged from hospital, and covering the first 12–24 months, which are the most crucial to prevent recurrent events.
Results
Discharge letter and instructions for patients
Patient follow-up after ACS is crucial to avoid a premature recurrent event and should involve a well-planned transition of care from the hospital to the patient’s cardiologist, general practitioner (GP) and any other associated healthcare providers. This process should involve a personalised and evolving approach to optimize clinical outcomes. An early ambulation (day 1) is usual for most STEMI or NSTEMI patients without residual ischaemic or heart failure clinical signs, and with no arrhythmic or mechanical complications. This mobilisation is facilitated by the implementation of radial access for PCI, and the hospital should provide a discharge letter following the standardized discharge letter after hospital stay [4].
The list of the information to include in the discharge letter is detailed in Table I .
Table I
Content of the hospital discharge letter
[i] ACE – angiotensin-converting enzyme, ALT – alanine aminotransferase, ARB – angiotensin receptor blocker, AST – aspartame aminotransferase, BMS – bare-metal stent, BVS – bioresorbable vascular scaffold, DAPT – dual antiplatelet therapy, DES – drug-eluting stent, GP – general practitioner, HAS – Haute Autorité de Santé, LVEF – left ventricular ejection fraction, OAC – oral anticoagulant, PCI – percutaneous coronary intervention.
Additionally, patients and their family members should be involved with discharge instructions about recognizing acute cardiac symptoms as well as the clinical signs of transient ischaemic attack or stroke. They also should be provided with clear instructions detailing lifestyle changes and their medication at discharge, including possible side effects and the risks associated with premature discontinuation of treatments [4, 5]. They must be aware that any bleeding does not systematically imply treatment cessation and they must understand what nuisance bleeding is. The letter should be given to the patient before discharge, even during weekends or on public holidays.
Management of ACS after discharge from hospital
An effective and coordinated evidence-based outpatient care plan – encompassing scheduled follow-up, appropriate personalised dietary and physical exercise recommendations, information on smoking and alcohol cessation, and adherence to treatments for secondary prevention [6] – is crucial for improving outcomes after an ACS. Timely follow-up is a key component of a transitional care model that reduces hospital readmission rates [7].
Transition care: the cardiologist and the GP
Both cardiologists and GPs play important roles in the long-term follow-up of patients, with regular reassessment of ischaemic and bleeding risks, adherence to treatment, management of adverse reactions, comorbidities and risk-factor management. At every stage of the follow-up, the shared medical decision with the patient and family members is key to reduce the residual risk.
The medical strategy will depend upon the patient’s stage of illness and life expectancy, the presence of comorbidities (e.g. chronic kidney disease, atrial fibrillation, diabetes mellitus or impaired cognitive functions), and the regional organization. The cardiologist plays a key role in the development of a comprehensive global risk-management strategy, the definition of treatment goals, the control of cardiovascular risk factors and the management of clinical events (Table II).
Table II
Checklist (cardiologist or general practitioner) for follow-up consultations after acute coronary syndrome
The role of the GP is obviously crucial due to the intimate knowledge and understanding of the patient. GPs can deal with early adverse reactions and be responsible for signalling the early signs of disease progression. GPs and cardiologists, in a strict collaborative fashion, are responsible for treatment adjustments and the management of major serious events. There is a great need for improved collaboration and communication, routine use of local networks, and shared patient files. Telemedicine may improve patient management.
Follow-up schedule
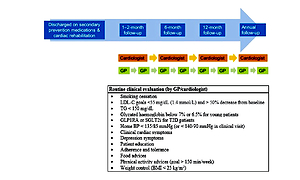
According to the AFSSAP (French National Health Agency) regular follow-up after ACS is recommended by both the cardiologist and the GP [8]. Based on the guidelines, in order to be on target as early as possible, the first meeting should be 4–6 weeks and the second one in another 4–6 weeks to rapidly reach the LDL-C goals [8]. This approach should be complemented by regular follow-up visits to the GP, ideally on a 3-monthly basis, especially in the presence of associated non-cardiac illness. The timing of consultations and actions required are illustrated in Figure 1.
Figure 1
Recommended follow-up after discharge for patients with ACS. Adapted from the French Haute Autorité de Santé recommendations [5]
Follow-up during the 1–6-month post-ACS period
During early follow-up consultations, the cardiologist and/or GP and patient can discuss activities such as return to driving and/or work, sexual and physical activities, cardiac rehabilitation and other quality of life measures.
The early consultation provides an ideal time to check for – and manage – any adverse reactions (e.g. myalgia, nuisance bleeding or tiredness) that can reduce adherence to treatment [9, 10].
This first contact also gives the opportunity to reinforce the importance of continuing secondary prevention measures, optimizing risk factor control and therapeutic goal achievements, adopting a healthy lifestyle, and appropriate education about the disease. Among patients with pre-discharge left ventricular ejection fraction (LVEF) ≤ 40%, repeat echocardiography 6–12 weeks after revascularization and optimal medical therapy is recommended to assess the potential need for an implantable cardioverter defibrillator (ICD) [11].
Follow-up during the 6–12-month post-ACS period and beyond
By 6 and 12 months, the patient’s ischaemic and haemorrhagic risks should be re-assessed to determine the duration of DAPT [12, 13]. Follow-up activities should be consistent with those outlined during the post-discharge consultations. In addition, as in early consultations, any contact with the patient is an opportunity to reinforce the importance of continuing secondary prevention measures and optimizing risk factor control. If the goals are not achieved, emphasis should be placed on optimizing the treatment and starting the combination therapy, so the patients achieve the goals as early as possible [6].
Ischaemic tests (e.g. stress imaging, exercise testing) are recommended if recurrent symptoms occur (Table III). The systematic use of ischaemic tests remains debated, requiring a dedicated randomized controlled trial (RCT). In case of doubts about the presented symptoms, the diagnosis of microvascular coronary disease should be considered.
Table III
Tests to be performed during follow-up after percutaneous coronary intervention for an acute coronary syndrome
Use of recreational drugs (e.g. cannabis, cocaine) that promote coronary thrombus formation and vasospastic angina [14] is widespread among young patients and warrants identification to ensure appropriate management. The psychosocial profile should also be assessed to prevent burn-out and depression [5].
Global atherosclerotic disease investigation and imaging should not be systematically performed according to current guidelines, even if it may be discussed, as the ankle-brachial index test is rapid, cheap, and provides additional information about the cardiovascular (CV) risk. The current strategy is to perform additional examinations following a clear rationale (Table III).
Treatment strategy
The European Society of Cardiology (ESC) 2017 STEMI guidelines recommended a ‘BASI’ approach to secondary prevention therapy after an acute myocardial infarction (MI), in which all patients should receive a b-blocker, an antiplatelet, a statin, and an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) [15]. The recommendations on secondary prevention therapies have since evolved, and the BASI approach is no longer applicable to all patients with acute MI, especially for β-blockers and ACE inhibitors/ARBs. It refers also to antihypertensive therapy and lipid-lowering therapy, when upfront combination therapy is recommended for those patients who are at the highest cardiovascular risk (including those with so-called extremely high cardiovascular risk) [16–18]. The routine secondary prevention therapies for ischaemic heart disease are detailed in Table IV [1, 2, 16].
Table IV
| Drug class | Treatment | Class and level |
|---|---|---|
| Antiplatelet | Indefinite treatment with low-dose aspirin (75–100 mg/day), in the absence of contraindications | IA |
| Clopidogrel in patients intolerant of aspirin | IB | |
| DAPT (aspirin + P2Y12 inhibitor (see below)), in the absence of contraindications, in patients treated with PCI | IA | |
| DAPT for up to 12 months, unless there are contraindications: | IC | |
| P2Y12 inhibitor in addition to aspirin for > 1 year after assessment of the patient’s ischaemic and bleeding risks | IIb | |
| DAPT for up to 1 year in patients without a stent | IIa | |
| In patients with a clear indication, OAC (VKA or NOAC) in addition to antiplatelet therapy NOAC | IC | |
| OAC | DOAC should be preferred. Duration of DAPT should be minimized (7 days) to reduce bleeding risk | IC |
| SGLT2 inhibitors | Patients with heart failure with reduced ejection fraction (HFrEF), diabetes, chronic kidney disease (CKD) | |
| ACE inhibitor or ARB | An ARB as an alternative to ACE inhibitor in patients with heart failure or LV systolic dysfunction, particularly for patients intolerant of ACE inhibitors | IB |
| All patients without contraindications | IIaB | |
| Vericiguat | Patients with heart failure or LV dysfunction (LVEF ≤ 40%) | IA |
| Lipid-lowering therapy (LLT) | Initiation of high-dose statins in patients without contraindications or history of intolerance, regardless of initial cholesterol values | IA |
| Addition of further lipid-lowering therapies to statin therapy if the LDL-C target is not achieved (< 1.4 mmol/l (55 mg/dl)) with the highest tolerated dose of a statin | IIaA | |
| If the LDL-C target is not achieved with the highest tolerable dose of a statin and ezetimibe, a PCSK9 inhibitor is recommended on top of lipid-lowering therapy; or alone or in combination with ezetimibe in statin-intolerant patients | IA | |
| In post-ACS patients with (1) extreme cardiovascular risk, (2) familial hypercholesterolaemia, or (3) baseline LDL-C concentration that prevents achievement of the treatment goal with statin therapy, upfront combination therapy with ezetimibe may be considered. | IIbC | |
| NSTEMI-ACS [2] (class and level of recommendation) | STEMI [1] (class and level of recommendation) | |
| Ticagrelor (90 mg bid) | Moderate to high risk cardiovascular risk patients without contraindications,a regardless of initial treatment strategy and including those pre-treated with clopidogrel (IB) | Ticagrelor preferred over clopidogrel (IA) |
| Prasugrel (10 mg (5 mg in patients < 60 kg)) | Patients planned for PCI (IB) without contraindicationsb Not recommended for patients with unknown coronary anatomy (IIIB) | Prasugrel preferred over clopidogrel (IA) |
| Clopidogrel (75 mg) | Patients who cannot receive ticagrelor or prasugrel or who require OAC (IB) | Preferably when prasugrel and ticagrelor are not available or are contraindicated (IC) |
[i] ACE – angiotensin converting enzyme, ACS – acute coronary syndrome, ARB – angiotensin receptor blocker, bid – bis in die (twice daily), DAPT – dual antiplatelet therapy, LDL-C – low-density lipoprotein cholesterol, LV – left ventricular, LVEF – left ventricular ejection fraction, NOAC – non-vitamin K antagonist oral anticoagulant, NSTEMI – non-ST-segment elevation myocardial infarction, OAC – oral anticoagulant, PCI – percutaneous coronary intervention, PCSK9 – proprotein convertase subtilisin/kexin type 9, STEMI – ST-segment elevation myocardial infarction, VKA – vitamin K antagonist. aPrevious intracranial haemorrhage or ongoing bleeds. bPrevious intracranial haemorrhage, ischaemic stroke or transient ischaemic attack or ongoing bleeds; prasugrel is generally not recommended for patients ≥ 75 years of age or with a bodyweight < 60 kg.
Antithrombotic treatment
Ischaemic and haemorrhagic risk assessment should be a dynamic process, as these evolve separately over time. This is the main reason why short- and long-term follow-up is necessary. We also advocate an adaptive approach, in response to the occurrence of an ischaemic or bleeding event, which may warrant a transient interruption.
Dual antiplatelet therapy
ESC guidelines 2017 (STEMI) and 2020 (NSTEMI) recommend that ACS patients should receive DAPT (comprising aspirin and a P2Y12 inhibitor) [2]. Currently, a 12-month DAPT therapy is considered as the default strategy in accordance with the results of several major trials [2]. As “one size doesn’t fit all”, the duration of DAPT should be individualized according to the benefit–risk ratio and adapted to events. Three oral P2Y12 inhibitors are available for the prevention of ischaemic events (Table IV): ticagrelor and clopidogrel are indicated for all types of ACS, whereas prasugrel is indicated for clopidogrel-naïve ACS patients scheduled for PCI and without previous history of stroke [1, 2]. Supplementary Table SI provide some pharmacological properties of these drugs. Prasugrel and ticagrelor are more effective than clopidogrel for reducing major ischaemic cardiovascular events, but are associated with an increased risk of bleeding (Supplementary Table SII) [19–21] and are contraindicated in some groups [21–23]. Platelet function monitoring to adapt the dose or type of P2Y12 inhibitors should not be used in ACS patients [24]. The preferred choice of prasugrel in PCI patients with NSTEMI is a class IIb based on the ISAR REACT 5 results.
Long-term DAPT: 12 months and beyond
Numerous RCTs have evaluated the effect of longer- versus shorter-term DAPT in patients with ACS (see Supplementary Table SIII [25–41]). The PEGASUS-TIMI 54 study [37] supports longer-term use of DAPT beyond 12 months, with significant reductions in MI and stroke compared with aspirin alone, despite an increase in bleeding. The DAPT study [33], which compared 30 versus 12 months of DAPT (clopidogrel or prasugrel), supports longer-term use of DAPT beyond 12 months to reduce the risk of ischaemic events and stent thrombosis, but with an increased risk of bleeding and an increase of non-cardiovascular death and total mortality which has not been fully explained [33, 42]. The remaining studies detailed in Supplementary Table III, which were overall underpowered, reported no significant improvement for longer- versus shorter-term DAPT. The MASTER DAPT study advocates a 1 month duration in patients with high bleeding risk (HBR) but included a majority of chronic coronary patients.
According to various guidelines, extended DAPT beyond 1 year with ticagrelor should be considered for a minority of patients at high ischaemic risk without a major bleeding event or increased bleeding risk [2, 42].
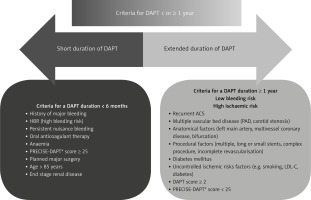
At 1-year follow-up, the DAPT score [43] may be helpful to guide the decision to continue DAPT beyond 12 months, but should complement, not replace, the clinician’s judgment. Factors to consider when identifying the optimum duration of DAPT are illustrated in Figure 2. The duration should be tailored to each patient’s ischaemic and haemorrhagic risk profile [44, 45], including the occurrence of events in the preceding period as well as their angiographic and clinical characteristics. Uncontrolled risk factors are obviously important to consider when estimating the risk of recurrent events [46]. The levels of ischaemic and haemorrhagic risk evolve over time, and thus require regular assessment of the benefit–risk balance.
Figure 2
Factors to consider when deciding on the optimal duration of dual antiplatelet therapy in patients with ACS. *For the PRECISE-DAPT score [17, 48]
ACS – acute coronary syndrome, DAPT – dual antiplatelet therapy, LDL-C – low-density lipoprotein cholesterol, PRECISE-DAPT – PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual AntiPlatelet Therapy.
As an alternative, the recent COMPASS trial has suggested that, in patients who require an aggressive long-term antithrombotic regimen, an association of low-dose aspirin and low-dose rivaroxaban may be considered [47].
Early discontinuation of DAPT after PCI for ACS
Discontinuation of the P2Y12 inhibitor after 6 or even 3 months may be justifiable in patients at high risk of bleeding [42]. The PRECISE-DAPT score [48] can be used to estimate haemorrhagic risk and informed discharge letter recommendations on treatment duration (Figure 2) [2, 7]. Patients reporting recurrent/persistent episodes of nuisance bleeding (but with low ischaemic risk) and those who require surgical intervention may be considered for single antiplatelet therapy rather than continuing DAPT (expert opinion). A recent study from Belgium reported that the most common reasons for stopping antiplatelet treatment before 11 months (among 295 ACS patients) were surgery (25%) and high bleeding risk (19%) [25].
In post-ACS patients who require surgical intervention, the risk of surgery-related bleeding must be balanced against that of recurrent ischaemic events related to interruption of antithrombotic therapy. This assessment should involve the type of surgery, patient’s ischaemic risk, time since the index event and PCI, and the risk of stent thrombosis. DAPT can then be discontinued or changed to a DAPT regimen with a lower bleeding risk (e.g. switch from aspirin plus prasugrel/ticagrelor to aspirin plus clopidogrel [49]). In patients who have had their DAPT regimen interrupted for surgery, this should be restarted after a period depending on surgery type and post-operative course. If aspirin is stopped, it is recommended to restart 24 h after low-bleeding-risk procedures or 48–72 h after higher-bleeding-risk procedures. While the maximal antiplatelet effect occurs within minutes after taking aspirin, the maximal antiplatelet effect of clopidogrel may not be reached until after 7 days of daily administration of a standard dose (75 mg/day) [50]. The antiplatelet effects are faster and more predictable with ticagrelor and prasugrel.
After stent implantation, elective surgery requiring discontinuation of P2Y12 inhibitors can be considered after 1 month, irrespective of the stent type, if aspirin can be maintained throughout the perioperative period (Class IIa; level B). In patients with recent MI, non-urgent surgery may be delayed until ≥ 6 months after the index MI event (IIb C). For patients in whom surgery cannot be delayed, DAPT should be continued in those considered to be at low bleeding risk and high ischaemic risk, whereas aspirin alone should be continued in patients at low ischaemic risk and high bleeding risk.
For non-cardiac surgery that cannot be delayed, a minimum of 1 month of DAPT is recommended [2].
New option of antiplatelet strategy after PCI for ACS patients
The TWILIGHT study enrolled 9,006 patients between July 2015 and December 2017 [51]. The patients were treated with PCI for ACS (64.8% of the population) or planned PCI. Trial inclusion criteria required the presence of at least one clinical and one angiographic feature associated with a high risk of ischaemic and/or bleeding events.
After 3 months of DAPT, event-free patients were randomly assigned to aspirin or placebo with continuation of ticagrelor for an additional 12 months. 7,119 patients were randomized in 11 countries (median age: 65.2 years old, 36.8% with diabetes mellitus, 23.8% females.). The primary endpoint was the rate of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding. The secondary endpoint was all-cause death, myocardial infarction, or stroke. The strategy with ticagrelor plus placebo was associated with an incidence of major bleeding of 4.0% for patients with 3 months DAPT versus 7.1% among patients who received ticagrelor plus aspirin for 12 months (hazard ratio (HR) = 0.56; 95% confidence interval (CI): 0.45 to 0.68; p < 0.001). Similar findings were reported for BARC 3–5 bleeding (1.0% vs. 2.0%; HR = 0.49; 95% CI: 0.33 to 0.74). Rates of all-cause death, myocardial infarction, or stroke were 3.9% for both groups (HR = 0.99; 95% CI: 0.78 to 1.25; pnoninferiority < 0.001). The rates of all-cause death (1.0% vs. 1.3%), myocardial infarction (2.7% vs. 2.7%), and definite or probable stent thrombosis (0.4% vs. 0.6%) were also similar between groups. No heterogeneity was observed irrespective of the ischaemic risk of the prespecified subgroups. The TWILIGHT study demonstrated that a shorter duration of DAPT (3 months) followed by single antiplatelet therapy (SAPT) with ticagrelor bid, compared to the recommended DAPT of 12 months’ duration, provides a 44% relative risk reduction of BARC 3–5 bleeding, with a similar rate of ischaemic events, and with a consistent effect in all ischaemic risk profiles. These findings will be considered in the next guidelines to optimize the antiplatelet strategy, and a DAPT of 3 months followed by ticagrelor bid in monotherapy seems a valid option by reducing bleeding risk without increasing ischaemic risk.
Triple antithrombotic therapy
Current guidelines for the management of patients on long-term oral anticoagulant (OAC) therapy undergoing coronary stenting are based on expert consensus [2, 52–54]. This frequent clinical situation of complex patients requires a collaborative decision involving the patient. The recent ESC guidelines suggest [2]: a limited period of triple antithrombotic therapy (OAC plus aspirin and clopidogrel) for as short a time as possible (7 days) irrespective of the type of stent used (IIa B); triple therapy for > 1 month and up to 6 months should be considered in high ischaemic risk patients due to ACS or other anatomical/procedural characteristics that outweigh the bleeding risk (IIa B); dual therapy with clopidogrel and OAC should be considered as an alternative to 1 month of triple therapy in patients in whom the bleeding risk outweighs the ischaemic risk (IIa A); after 12 months, discontinuation of all antiplatelet therapy should be considered (pursue OAC alone) (IIa B) [55]. Use of a direct OAC is preferable to a vitamin K antagonist (warfarin only). Prasugrel and ticagrelor should not be used in the triple combination due to excess risk of bleeding. An antiplatelet agent may be prolonged for high-risk thrombotic situations (left main and/or multiple stenting, recurrent ischaemic events, or previous stent thrombosis). The ESC STEMI guidelines advised that OAC should be considered for up to 6 months once the thrombus is identified, guided by repeated echocardiography and with continuous evaluation of bleeding risk and the need for concomitant antiplatelet therapy. The optimal duration of OAC in these patients remains unclear; therefore decisions regarding the OAC duration should be individualised.
Lipid-lowering therapy
Based on the guidelines, all ACS patients without contraindications, regardless of their low-density lipoprotein cholesterol (LDL-C) level, should be started on high-dose statin (atorvastatin 40–80 mg or rosuvastatin 20–40 mg) therapy during hospitalization. For US guidelines, the LDL-C target is below 70 mg/dl, whereas ESC guidelines consider a lower level below 55 mg/dl, due to the proven benefits reported by recent RCTs, with a reduction of ≥ 50% from baseline (if > 1.8 mmol/l) [56–62]. It needs to be emphasized that ESC/EAS 2019 guidelines [56] also introduced the extremely high-risk category, for those with 2 vascular events in the last 2 years, which was next extended in different national and international recommendations, for which the targeted level of LDL-C should be < 40 mg/dl (1 mmol/l). It is important as secondary prevention patients at very high risk are a very heterogenous group [63].
Dose adjustment and the addition of ezetimibe on top of the maximally tolerated statin dose are necessary in patients whose LDL-C value remains above the goal [56–58]. The protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (evolocumab and alirocumab) should be considered (based on the reimbursement criteria) if patients are not at LDL-C goal (above 70 mg/dl) with the lipid-lowering combination statin and ezetimibe. Inclisiran was approved by the European Medicines Agency (EMA) in December 2020 twice a year (3 times a year in the first year of the therapy) and will be a great complement to the PCSK9 inhibitor therapy. For patients at extremely high cardiovascular disease (CVD) risk and those with very high baseline LDL-C levels that do not allow them to be on goal with statin monotherapy (assuming about 50% LDL-C reduction) upfront lipid-lowering combination therapy with statins and ezetimibe should be considered [14, 18].
Based on the most recent data, the worldwide prevalence of statin intolerance is 9.1% [59–64]. Patients who report symptoms of statin intolerance should always be diagnosed based on the approved definitions [9] assessed using the statin associated muscle symptoms clinical index (SAMS-CI) [65]. Recent data suggest that nocebo effects might be responsible for even 50–70% of the cases [66]. The ESC/European Atherosclerosis Society (EAS) have published joint recommendations for the management of myalgia with statins using a clinical algorithm [9, 64]. Bempedoic acid, besides innovative drugs associated with PCSK9 inhibition, alone or in fixed combination with ezetimibe, is a very effective option in patients with confirmed statin intolerance [9, 67].
Other secondary prevention medications
The systematic use of β-blockers and ACE inhibitors/ARBs in all ACS patients is no longer recommended [2], and physicians should consider stopping these treatments in patients without clear indications. β-Blockers should not be administered in patients with symptoms possibly related to coronary vasospasm or cocaine use [2].
ESC guidelines state that ACE inhibitors are recommended (IA) in patients with heart failure, left ventricular systolic dysfunction, diabetes mellitus or renal failure [1, 2]. An ARB can be used as an alternative in patients with heart failure and/or left ventricular systolic dysfunction, and in patients who are intolerant of ACE inhibitors. Mineralocorticoid receptor antagonists (MRA) are also a therapeutic option in patients with left ventricular dysfunction, symptomatic despite optimal medical therapy.
Long-term treatment with oral β-blockers is recommended, in the absence of contraindications, for all patients with STEMI [1] and for non-ST-elevation ACS patients with LVEF ≤ 40% [2]. Their long-term benefit in patients with preserved ejection fraction or LVEF > 40% remains uncertain and several trials are ongoing to determine the best option [1, 2].
In patients with diabetes, glycaemic control with a haemoglobin A1c goal < 7% should be encouraged, because it has been associated with a reduction in the risk of incident MI [46] or non-fatal MI.
Cardiac rehabilitation
Patients, and family members, should be educated and actively involved for lifestyle changes and risk factor management (Table II) [1, 2, 16, 59, 65, 67]. Counselling should start during hospitalization and continue at discharge and during follow-up [68]. Strategies to encourage healthy lifestyle changes and adherence to secondary prevention, such as attendance at a cardiac rehabilitation programme and joining a support group, should be offered [2, 4, 68, 69]. In the French FAST-MI registry, prescriptions for cardiac rehabilitation after acute MI were associated with improved 5-year survival rates, but were only offered to 22% of patients, indicating considerable room for improvement [68].
Regular attendance at cardiac rehabilitation is highly recommended, but depends upon the availability of dedicated centres and sufficient resources. Later sessions can be prescribed by cardiologists every 6 months if appropriate. Patients at the highest risk (e.g. those with LVEF ≤ 40%) and young patients who are most likely to return to active and/or professional life should be prioritized.
Leisure-time physical activity and competitive sports
In the ESC guidelines [69], suitable physical activity is encouraged in patients with coronary artery disease (CAD). Patients with higher cardiovascular risk profiles are not eligible for competitive sports but can participate in an individually designed physical activity, whereas those at lower risk are eligible for low or moderate static and low dynamic sports [69]. The benefits of regular physical activity outweigh the low risk of initiating a coronary event during the exercise session, but patients with CAD should be given instructions on appropriate activities to minimize risks and maintain a safe level of intensity [69]. Patients who have undergone PCI should perform an exercise test before they resume physical activity. After completion of outpatient cardiac rehabilitation – usually 4–6 weeks after the index event – patients who are asymptomatic may resume a programme of individually tailored physical activity under the supervision of a qualified physician [69]. There are also recommendations available concerning the statin therapy in patients with intensive exercises and athletes in order to avoid SAMS and statin intolerance [70].
US guidelines on sport in competitive athletes [70] state that asymptomatic patients with CAD, without inducible ischaemia or electrical instability, can reasonably participate in all competitive activities if their resting LVEF is > 50%. For those with a lower LVEF, it is reasonable to restrict them to sports with low dynamic and low–moderate static demands. Patients with clinically manifest CAD should be prohibited from participating in competitive sports for ≥3 months after an acute MI or coronary revascularization procedure.
According to French law (Article L1172-1), sport can now be ‘prescribed’ in the context of public health. The objective of the prescription is to provide 3 months of support (from a massage therapist, occupational therapist, teacher in adapted physical activity, sports educator or non-graduate trained volunteer) and follow-up by a teacher in adapted physical activity, to lead towards autonomous and long-term activities on the part of the patient.
Smoking cessation
Smoking cessation is absolutely crucial but remains suboptimal in the EUROASPIRE surveys [71]. Nicotine-replacement patches, which have been shown to be safe in ACS [72], can be recommended to aid stopping smoking and can be started during hospitalization. Electronic cigarettes (which deliver a nicotine-containing vapour) are generally perceived as a ‘healthier alternative’ to conventional cigarettes, but no data exist to demonstrate their comparative safety, their efficacy in reducing tobacco dependence or their potential cardiovascular effects [73–75].
Quality of life
Quality of life is key, with the goal being to be able to return to normal daily activities. Guidance for resumption of daily activities must be based on LVEF, revascularization success, rhythm control and job characteristics (if in employment) [1]. Sexual activity is reasonable 1 week after an uncomplicated MI in asymptomatic patients during mild to moderate physical activity (IIa C) [68], but this should be adjusted based on physical ability (threshold ≥ 3 metabolic equivalent of task). Driving can be restarted in accordance with each country’s law. In France, patients should be stabilized (about 5 days if LVEF > 50%; otherwise about 4 weeks after the index event for personal driving, 6 weeks for professional drivers, after specific evaluation) [76–78].
No restrictions are necessary for long-distance air travel in asymptomatic patients. For patients with complicated STEMI, travel should be deferred until the patient’s condition becomes stable [1].
Return to work depends on each patient’s profile and previous activity. A simple algorithm [79, 80] represents a useful tool to help physicians. Guidelines encourage a return to work 1–3 months after an ACS, but this obviously depends on the individual.
Discussion: this expert position aims to help healthcare professionals in daily practice, as transition care and follow up are complex, as underlined by the high rates of ischaemic and bleeding events reported by several registries. As some gaps of evidence remain and need further research, our advice represents a practical guide that should be adapted to the patient’s characteristics and preferences, as well as regional access to healthcare.
Conclusions
Follow-up after an ACS represents a crucial challenge, due to the high residual ischaemic risk, potential bleeding, to fight therapeutic inertia, and reinforce therapeutic education. Therefore, optimal management should be personalised, reactive and adaptive to clinical situations (recurrent ACS, bleeding events, surgical procedures). The paternalistic model is no longer valid and should be replaced by a shared decision-making approach. Follow-up is based on a patient-centred approach, involvement of the patient and family members and collaboration between health professionals to optimize long-term management. Checkpoints seem crucial during the first month, at 3 or 6 months (to shorten the DAPT), and then at 12 months. The 1-month visit makes it possible to manage nuisance bleeding, to explain the benefits of treatments, to titrate lipid-lowering treatments if needed, and to repeat educative key messages to patients in order to maintain long-term adherence and compliance. The 3-and/or 6-month visit also allows further optimization of lipid-lowering therapy (if necessary) and a risk–benefit evaluation of DAPT in frail patients, in which it can be replaced by SAPT. The 1-year evaluation by the cardiologist aims to identify the minority of patients who may benefit from prolonged DAPT or a combination of SAPT with low-dose rivaroxaban. Patients with multivessel coronary disease and/or polyvascular disease and/or persistent non-controlled risk factors (smoking, diabetes, dyslipidaemia) with a low bleeding risk seem the best candidates. There is no perfect risk score, but the DAPT score may help to the decision. In the near future, cognitive computing may become an effective tool to improve and refine the current scores, and should be used at the point of care, tailored to individual patient characteristics. Long-term care by cardiologists improves patient adherence to secondary prevention strategies and lifestyle changes. Healthcare providers must be focused on the patient’s individual profile and personal level of risk, and should adapt the strategy every time it is required. Ongoing trials and registries will provide further information on the best approach for frail patients and/or in complex situations.