The Oxford-AstraZeneca (AZ) COVID-19 vaccine is a viral vector vaccine containing a modified, replication-deficient chimpanzee adenovirus ChAdOx1 [1]. The COVID-19 vaccine has been the most effective public health intervention in combating the current COVID-19 pandemic. Though the vaccine appears to be safe and is recommended even for patients with nephrotic syndrome, a few cases of relapsed nephrotic syndrome have been reported. Here we report a first case of relapsed focal segmental glomerulosclerosis (FSGS) and severe acute kidney injury (AKI) supported by renal replacement therapy after receiving the AZ COVID-19 vaccine.
A 54-year-old Asian male with history of asthma, gouty arthritis, thalassaemia, and dyslipidaemia was diagnosed with FSGS, which was confirmed by renal biopsy in April 2014. During initial diagnosis of fist FSGS, his serum creatinine was 2.3 mg/dl and his urine protein-to-creatinine ratio (UPCR) was 11.22. He did not take non-steroidal anti-inflammatory agents after biopsy. Viral infections such as HIV and illicit drug use such as heroin were excluded. He received prednisolone and cyclosporine for 12 months and was then changed to prednisolone and mycophenolic acid for 2 months. After treatment, serum creatinine was 1.5 g/dl and UPCR became undetectable. During a follow-up period of 5 years, his health status remained good without using immunosuppressive agents. Meanwhile, his serum creatinine gradually increased from 1.5 to 2.1 and UPCR was still undetectable.
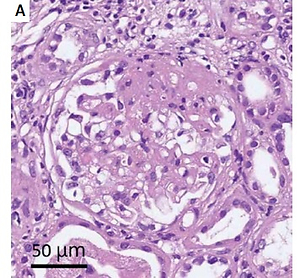
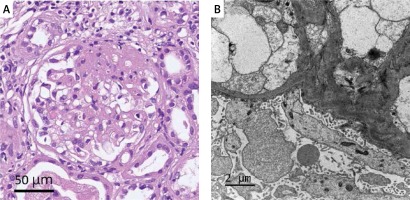
This patient received 2 doses of AZ COVID-19 vaccine in August and September 2021. One month later, bilateral leg oedema, fatigue, and poor appetite were noted, and he presented to our outpatient department for help. Blood and urine examinations were done, revealing a blood urea nitrogen (BUN) of 96.9 mg/dl, creatinine of 9.60 mg/dl, and serum albumin level of 2.5 g/dl. UPCR was 23.0 g/g. Viral infections were excluded. His COVID PCR result was negative. Anti-glomerular basement membrane (GBM) antibodies, and the antineutrophil cytoplasmic antibodies (ANCA), namely anti-proteinase-3 (PR3), and anti-myeloperoxidase (MPO) were negative. The antinuclear antibodies, anti-SSA/SSB antibodies (antiRo/La Ab), anti-Scl 70 antibody, and anti-Sm and RNP antibody were all negative. A renal biopsy was performed (Figure 1). Light microscopy showed FSGS superimposed upon a background of tubulointerstitial nephritis, and moderate interstitial fibrosis, and tubular atrophy. Electron microscopy showed widespread effacement of glomerular epithelial cell foot processes. No evidence was found of an immune complex-mediated renal injury process. Compared with his previous renal biopsy 5 years prior, the degree of interstitial fibrosis and tubular atrophy increased from 10% of cortical parenchyma in the initial biopsy to 30% in the subsequent biopsy. The degree of total inflammation was minimal (less than 5%) in the initial biopsy, but more prominent tubulointerstitial inflammation (about 40%) was identified in the subsequent biopsy. Accompanied by the clinical manifestations of nephrotic syndrome, the ultrastuctural findings in glomerular capillary walls showed widespread effacement and microvillous transformation of podocyte foot processes. The above features suggest changes of podocytopathy, and the patient’s FSGS can be classified as primary.
Figure 1
A – Light microscopy shows focal segmental glomerulosclerosis, tubulointerstitial nephritis over background, moderate arteriosclerosis, mild arteriolar hyalinosis, moderate interstitial fibrosis, and tubular atrophy. B – Electron microscopy shows focal segmental glomerulosclerosis and widespread effacement of glomerular epithelial cell foot processes
The patient received prednisolone after biopsy but experienced progressive dyspnoea, and his creatinine increased markedly to 16.42 mg/dl. Due to severe AKI, he received emergent haemodialysis. During a 2-month follow-up, his condition became stable after regular haemodialysis and prednisolone use. Laboratory examinations revealed a creatinine of 5.20 mg/dl and serum albumin level of 2.9 g/dl. UPCR was 10.31 g/g.
According to previous studies, the “vaccine” has been a potential trigger factor for dramatic immunologic response. Sporadic cases of de novo/relapse of nephrotic syndrome developing after influenza virus, hepatitis B, pneumococcus, meningococcal B, smallpox, measles, etc. have been reported [2]. To the best of our knowledge, few studies have reported cases that developed de novo/relapse nephrotic syndrome after the Janssen, Pfizer-BioNTech, and Moderna COVID-19 vaccines [3, 4]. As regards the Oxford-AstraZeneca vaccine, a case report [5] described a patient who developed de novo minimal change disease (MCD) with severe AKI 2 weeks after receiving the first dose. Another study [4] reported an MCD patient who had been in complete remission for 2 years but was then diagnosed with relapse of primary FSGS (tip-variant) 3 weeks after the Pfizer-BioNTech vaccine. However, the overall immunological impact of the Oxford-AstraZeneca (AZ) COVID-19 vaccine on the relapse of FSGS is still unknown.
This case report provides clinical information about a case of FSGS relapse with severe AKI injury after COVID-19 vaccination. Patients with glomerulonephritis should be advised about the risk of relapse before vaccination and clinicians need to consider follow-up renal function and proteinuria after vaccination.