This editorial refers to ‘A worsened prognosis for patients after stroke with newly diagnosed atrial fibrillation compared to having a preexisting arrhythmia’, by Tracz J., et al., https://doi.org/10.5114/aoms/152339 and ‘Trends in stroke prevalence caused by an elevated body mass index in the European population’ by Tao M., et al., https://doi.org/10.5114/aoms/152339 and ‘Trends in stroke prevalence caused by an elevated body mass index in the European population’ by Tao M., et al., https://doi.org/10.5114/aoms/199362.
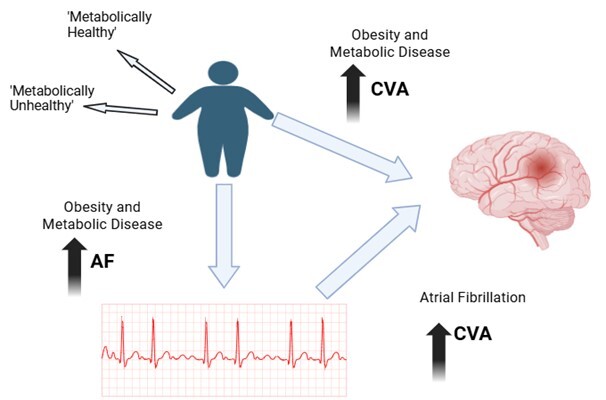
Obesity is a global public health problem, and there is good evidence to show that it is linked to not only an increase in the incidence of the commonest cardiac rhythm disorder, atrial fibrillation (AF). Moreover, obesity not only raises the risk of developing AF but also contributes to its progression and recurrence. Both obesity and AF are major risk factors for stroke [1]. Obesity also indirectly heightens the likelihood of experiencing ischemic stroke due to related health issues such as high blood pressure and metabolic diseases [1]. As the prevalence of obesity and cardiometabolic diseases rises, the incidence of AF appears to be increasing as well [2, 3]. The relationship between obesity, AF, and stroke is complex, involving both direct and indirect mechanisms [1].
Furthermore, obesity is not a binary condition; it encompasses various phenotypes, and obesity-related outcomes are influenced by several factors, including race and metabolic health. For instance, metabolically healthy obese patients with AF do not face a higher risk of adverse outcomes [4, 5]. In fact, metabolically healthy obese patients have the same risk of adverse major adverse cardiovascular events (including stroke) compared to metabolically healthy non-obese individuals, but may still have a higher risk of new-onset HF and new-onset AF [6].
For both clinical and research purposes, body mass index (BMI) is the most used anthropometric measurement of obesity. However, it does not reflect body composition, the distribution of weight, or racial differences in fat distribution. Alternative measurements, such as the Body Roundness Index (BRI), Waist-to-Hip Ratio (WHR), and radiographically derived MRI from DEXA scans, may provide a better understanding of body composition and distribution [7]. Additionally, BMI does not always reflect an individual’s underlying metabolic health accurately.
Nevertheless, things may not be so straightforwards. The ‘obesity paradox’ refers to the observation that obese patients with cardiovascular diseases sometimes achieve better outcomes than their thinner counterparts [8, 9]. While this theory is not universally accepted, various studies and cohorts have reported such findings [8]. There are three main explanations for the obesity paradox: 1. Some obese individuals may develop metabolic adaptations that enhance their ability to cope with stress. 2. Another adaptation observed in obese individuals is a higher left ventricular ejection fraction (LVEF). 3. Lastly, selection bias and confounding factors may also contribute to these unexpected results [10].
This edition of the Archives of Medical Science features two papers that focus on the relationships between obesity, AF and stroke. Tracz et al. [11] report on a worsened prognosis for stroke patients with newly diagnosed AF compared to those with preexisting arrhythmia [11]. This was a single-centre retrospective study involving a cohort of 2,000 individuals who experienced acute ischemic strokes. Among these patients, AF was newly diagnosed in 123 (21.2%) individuals, referred to as the ‘new-AF’ group, while 456 (78.8%) patients had a history of AF, termed the ‘previous-AF’ group [11]. Stroke severity, as measured by the National Institutes of Health Stroke Scale (NIHSS) score, was higher in the new-AF group than in the previous-AF group, while mean CHA2DS2VASc before hospitalization were lower in the new-AF group compared to the previous-AF group [11].
Within the overall study population, thrombolysis was employed in 7.6%, more commonly (12.2%) from the new-AF group and only 6.4% from the previous-AF group (p < 0.048). Although there were no significant differences in the rates of haemorrhagic transformation, thrombolysis is not recommended for patients who are anticoagulated [11] and one might assume that the previous-AF patients who received thrombolysis had a CHA2DS2VASc score low enough to avoid anticoagulation requirements or were not anticoagulated. Another limitation of the study by Tracz et al. [11] was the difficulty in obtaining detailed information regarding thromboembolic prevention, treatment prior to hospitalization, and the history of anticoagulant and antiplatelet treatments in a significant number of participants. In-hospital mortality was notably higher in the new-AF group (10.6%) compared to the previous-AF group (3.5%). Differences in comorbidities between the new-AF and previous-AF cohort were apparent.
There is also some conflicting evidence with regards to whether new-onset AF results in increased stroke severity. One retrospective study of 196 patients with acute ischaemic stroke, found that after propensity score matching, stroke severity and in-hospital outcomes in patients with newly diagnosed AF did not differ from those in patients with known AF [12].
Nevertheless, AF can be asymptomatic, and it is difficult to determine the temporality of when AF started in most patients. Therefore, for diagnostic purposes new-onset AF is essentially regarded as the same as first-detected AF. In reality, there is heterogeneity in those labelled as new-onset AF and increased AF burden is associated with an increased risk of stroke and thromboembolic events [13]. In AF diagnosed after stroke (AFDAS), there is some evidence to show that AF detected on a prolonged cardiac monitor (PCM-detected AF) carries a lower risk of stroke, and that ECG-detected AF may be associated with a 5-fold higher adjusted recurrent ischemic stroke risk [14, 15]. Hence, the method of diagnosis is important, and detection yields increase with longer and more sophisticated monitoring but are not necessarily accompanied by a decrease in mortality [16].
The second paper in this issue uses European stroke and BMI data from the Global Burden of Disease (GBD) database (1990–2019) to examine the relationship between stroke and elevated BMI in individuals aged 20 to 94 [17], defining an elevated BMI and obesity as a BMI greater than 25 kg/m2. Of note, the WHO defines a BMI > 25 kg/m2 as overweight, a BMI > 30 kg/m2 as obese, and a BMI ≥ 35 kg/m2 as advanced obesity [18]. Some studies show that extremely obese and underweight individuals may have increased mortality rates and worsened functional outcomes, whilst overweight individuals may have better outcomes [19, 20].
One of the main findings from this study was that stroke mortality rates were associated with elevated BMI. The analysis of the period effect revealed that the risk of stroke mortality associated with an elevated BMI initially increased, then decreased, and later increased again over the years; however, the overall trend was not statistically significant [17]. For individuals aged 80 to 84, the risk of stroke mortality due to an elevated BMI gradually declined with age, suggesting that a higher BMI may offer some protective benefits in older age groups. This study did not clearly observe an obesity paradox (except perhaps for the elderly subgroup) and was unable to investigate more complex factors linked to this such as sociodemographic variables and fat distribution.
Tao et al. also found that earlier birth cohorts had a significantly higher relative risk (RR) of stroke mortality [17]. The RR for stroke death in males born between 1910 and 1914 was 8.29-fold higher than in males born between 1995 and 1999 [17]. The period effect showed that the risk of stroke mortality caused by an elevated BMI in Europe showed an insignificant trend of generally rising [17]. The variation in stroke mortality risk in elevated BMI amongst different cohorts illustrates that biological factors, such as metabolic health and comorbidities, as well as socioeconomic factors and the availability and advancement of treatment, significantly influence stroke mortality. Indeed, one limitation of the GBD database is a lack of granular data with respect to socioeconomic factors and comorbidities.
Hence, there were changes due to the period effects, but the changes related to cohort effects were more pronounced. This suggests that factors influencing stroke risk, including both medical and socioeconomic factors have the potential to have a more significant impact on stroke mortality. Additionally, these influences may be more significant across different cohorts compared to temporary events specific to a particular period.
What are the implications? This means that to effectively reduce stroke mortality risk related to obesity, public health interventions should be designed to address obesity, as well as co-morbidities and socioeconomic factors related to obesity. For prospective interventions it would be useful to understand which factors had the most significant impact on mortality. It would also be useful to know if these trends varied across European nations.
The current focus should be on holistic treatment of AF, including addressing obesity and associated metabolic conditions. AF screening and risk-based prediction algorithms should also be optimized to enhance detection rates and improve the cost-effectiveness of future screening programs for AF [21, 22]. Use of the evidence-based Atrial fibrillation Better Care (ABC) pathway provides a structured, integrated approach to improve patient outcomes, with three key elements: (A) Anticoagulation/Avoid stroke, (B) Better symptom management, (C) management of Cardiometabolic co-morbidities with optimal medical therapies and management of lifestyle factors to reduce obesity [23–25]. This allows for both primary care and secondary care to provide structured, holistic treatment of both a patient’s medical co-morbidities and lifestyle related factors. This approach has also been adapted for stroke, the post-stroke ABCstroke pathway, a collaborative approach to facilitate an effective and efficient integrated care service model [26, 27]. Future studies should move beyond binary definitions of obesity and comprehensively consider an individual’s underlying metabolic health.