Introduction
Steroid-dependent nephrotic syndrome (SDNS) is a class of refractory nephrotic syndrome. A patient with SDNS relapses during steroid taper or withdrawal. Moreover, maintaining high-dose treatment for long term causes severe immunosuppressive effects [1, 2], and increases morbidity and mortality [3, 4]. Other side effects caused by steroid treatment such as cushingoid features, hypertension, growth failure, and emotional problems are also reported [5].
Severe proteinuria and hypoproteinemia characterize the nephrotic syndrome, which is common in children and adults [6]. Rituximab (RTX), a chimeric monoclonal anti-CD20 antibody, is used in the treatment of several immune diseases [7–9], and recently it was observed to be efficacious in the treatment of pediatric and adult SDNS [10–15].
To explore the efficacy and safety of RTX treatment in patients with SDNS, we reviewed the literature regarding its therapeutic use in SDNS.
Material and methods
Literature search
Search terms were “rituximab” or “anti-CD20 antibodies” and “steroid-dependent nephrotic syndrome” or “SDNS” or “frequently relapsing nephrotic syndrome” or “FRNS”. Currently, RTX is permitted only in children with SDNS, so Clinical Trials were waived, and keyword searches were conducted in PubMed, EMBASE and Cochrane Library from interception to 2019-6-6, without language limitation. We considered only adult cases (age ≥ 19). Reference lists of every relevant trial or review article were also searched. Disagreement was resolved through discussion.
Inclusion and exclusion criteria
Included articles met the following criteria: 1) Diseases: SDNS or FRNS. 2) Treatment: RTX. 3) Patients’ age ≥ 19. Exclusion criteria: 1) Secondary treatment for other systemic diseases; 2) Combined treatment with other immunosuppressive drugs; 3) Patients’ age < 19.
Data extraction
Data from eligible studies were divided into two parts: 1) Basic information: the first author, publication year, PMID in PubMed, database identification number, number of patients, age of patients, administration of RTX, follow-up; 2) Outcomes data: clinical characterization (relapse, prednisolone dose, serum albumin, proteinuria and serum creatinine), immunological characterization (IgG, CD19 cell count, CD20 cell count, CD4/8 ratio, Th1/2 ratio). Adverse effect (AE) of prednisolone (body mass index (BMI), total cholesterol, diastolic blood pressure (DBP), systolic blood pressure (SBP), bone mineral density (BMD), and T-score of BMD), and AE of RTX.
Statistical analysis
Hypoalbuminemia, severe proteinuria, prednisolone dose and frequent relapse were characteristics of SDNS. So, relapse times, prednisolone dose and those laboratory indexes were extracted to estimate the efficacy of RTX in SDNS. Heavy and long-term treatment with prednisolone causes obesity, hyperlipidemia, hypertension and osteoporosis. Thus, we compared the BMI, blood total cholesterol, SBP, DBP, BMD and T-score to assess whether RTX reduced the adverse effect of prednisolone in patients with SDNS. RTX is a monoclonal antibody targeting CD20 positive cells. We reported the data regarding the level of IgG, CD19 cell count, CD4/8 cell ratio and Th1/2 cell ratio except CD20 cells to explore the immunological influence of RTX in patients.
Data were extracted to compare the pre- with the post-RTX treatment and management with the Review manager 5.3 software to calculate the mean differences. Data were continuous variables and were expressed as mean ± standard deviation (SD). Standardized mean difference (SMD) with 95% confidence intervals were used. We calculated I2 to estimate the heterogeneity of the included studies. When I2 < 50%, we used a fixed-effects model. In the case of I2 ≥ 50% to assume substantial variability, we used a random-effects model. We considered significant those differences with a p-value < 0.05.
Results
Search results and study characteristics
Among the 135 abstracts identified, 8 fitted our inclusion criteria with 209 patients and a male/female ratio of 143/66. The follow-up period was no less than 12 months. RTX was administered in a single dose of 375 mg/m2 at 6-month intervals or 1000 mg (Table I).
Table I
Basic characteristics of eligible studies
Rituximab was effective in the treatment of SDNS
Four studies [16–19] (102 patients) were included to assess the influence of RTX on disease relapse. The I2 was 0, so a fixed effects model was used. We found a statistically significant difference between pre- and post-RTX treatment relapse in SDNS patients after RTX was administered less frequently (SMD: –1.96 (–2.30, –1.62), p < 0.00001; Figure 1).
Four studies [16, 19–21] registered the influence of RTX on prednisolone dose. Since the I2 was 50%, moderate heterogeneity existed, and a random effects model was conducted. Total impact was –2.23 (–2.69, –1.77) with p < 0.00001 on the dose of prednisolone, showing statistical significance. So, the dose of prednisolone required was reduced after RTX administration (Figure 1).
Six studies [17–22] (173 patients) were included to estimate the influence of RTX on the level of serum albumin. The value of I2 was 97%, which showed substantial heterogeneity, then a random effects model was chosen. The SMD was 2.52 (0.95, 4.08) with p = 0.002, which showed that the level of serum albumin between baseline and post-RTX was statistically significant, and the level of serum albumin increased after RTX administration (Figure 1).
Four studies [17, 19–21] (134 patients) were considered to investigate the effect of RTX on proteinuria. Since proteinuria disappeared after RTX treatment in two studies [17, 19], these data could not be pooled for analysis. Proteinuria was ≤ 0.5 g/day, which was defined as a complete response in the treatment of nephrotic syndrome, and the level of proteinuria met the criteria in the other two studies [20, 21]. So, proteinuria was lower after RTX treatment (Figure 1).
Six studies [17–22] could evaluate the role of RTX in the change of serum creatinine. The value of I2 = 0% indicates no heterogeneity, so a fixed effects model was used. The SMD was –0.13 (–0.34, 0.09) with p = 0.25, which was more than 0.5. No statistically significant difference was detected, so the level of serum creatinine was undifferentiated between post-RTX and baseline (Figure 1).
Rituximab reduced the adverse effect of prednisolone
Three [18, 20, 22] of the eight quality studies were associated with BMI. Since no heterogeneity was found (I2 = 0%), we applied a fixed-effects model. The BMI after RTX treatment was similar to baseline (SMD = 0.01 (–0.29, 0.32); p = 0.93) (Figure 2).
Figure 2
Impact of RTX on the adverse effects of prednisolone
BMI – body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, BMD – bone mineral density.

Five studies [17, 18, 20–22] assessed the effect of RTX in the SBP and DBP. Both I2 = 0%, no obvious heterogeneity was shown, so a fixed effects model was conducted. The SMDs were –0.54 (–0.79, –0.29) with p < 0.0001 and –0.48 (–0.73, –0.24) with p = 0.0001 for SBP and DBP, respectively. Both had statistical significance, so after RTX administration, SBP and DBP were lower than baseline (Figure 2).
The influence of RTX on the total cholesterol was assessed in five studies [17, 19–22]. Large heterogeneity was observed in the included studies (I2 = 67%), so a random effects model was used. There was a statistically significant difference between post-RTX and baseline (SMD was –0.97 (–1.40, –0.54) with p < 0.00001), showing that total cholesterol was lower after RTX administration (Figure 2).
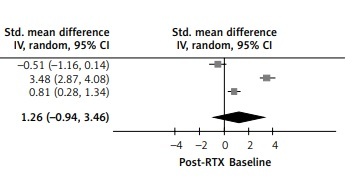
The impact of RTX administration was assessed by BMD [17, 19, 20] and T-score of BMD [18–20] in three studies. Both heterogeneities were large, for I2 = 92% and I2 = 98% in BMD and T-score of BMD, and random effects models were used. The SMDs were 1.26 (0.19, 2.34), p = 0.02 and 1.26 (–0.94, 3.46), p = 0.26 respectively. Statistical significance was observed in BMD but not T-score of BMD; the level of BMD increased after RTX was administered but not T-score of BMD (Figure 2).
Some immunological indexes were changed after RTX was administered
Four studies [17–20] evaluated the change of IgG. Overall, the I2 was 97%, which showed substantial heterogeneity, so a random effects model was conducted. The result of pooled data was 2.54 (0.53, 4.55) with p = 0.01, showing statistical significance for IgG levels (Figure 3).
Three studies [17, 19, 20] were included to analyze the change of CD20 and CD19 cells. Both I2 values (99%) justify the random-effects model. The pooled results were –4.90 (–8.88, –0.93) with p =0.02 and –4.52 (–8.42, –0.63) with p = 0.02 respectively, showing statistically significant differences, and CD20 and CD19 cell counts were lower than baseline, as expected (Figure 3).
Four studies [17–20] were about the change of CD4/8 after RTX was administered. The I2 was 97%, which means substantial heterogeneity, and a random-effects model was applicable. The pooled result was 1.42 (–0.31, 3.15) with p = 0.11, and no statistically significant difference was observed. CD4/8 ratio was similar to baseline after RTX treatment (Figure 3).
The effect of RTX on the change of Th1/Th2 ratio was favorable in three studies [17, 19, 20]. I2 = 0%, no obvious heterogeneity was detected, and a fixed-effects model was conducted. The result of pooled data was –6.74 (–7.70, –5.77) with p < 0.00001, and a statistically significant difference was observed between baseline and post-RTX. Th1/Th2 ratio was lower after RTX was administered (Figure 3).
Adverse effects of rituximab
The AEs [16–18, 21] of RTX are summarized in Table II. The most common adverse effect was infusion reaction, which occurs within 24 h after RTX administration. The long-term AEs were referable to hematologic reactions, such as leukopenia, neutropenia and agranulocytosis. Those AEs were mild, reversible or curable.
Table II
Adverse effects of rituximab
Discussion
Treatment of steroid-dependent nephrotic syndrome usually involves steroids accompanied by a broad array of immunosuppressants. The most commonly used immunosuppressive drugs are calcineurin inhibitors, alkylating agents, and anti-proliferative immunosuppressants. However, while alkylating agents and anti-proliferative immunosuppressants had compromised defense against viruses and malignancy [23], calcineurin inhibitors cause significant nephrotoxicity [24]. In our meta-analysis, we found RTX effective in the treatment of SDNS by reducing relapse, prednisolone dose, and proteinuria, increasing the level of albumin and having no impact on the serum creatinine. RTX was also able to reduce total cholesterol and blood pressure, and increase serum IgG. RTX alleviated some side-effects of prednisolone, by the reduction of prednisolone dose, and ameliorating the immune response and the lipid metabolism [18]. Ruggenenti et al. [22] also found that RTX treatment benefited blood pressure in pediatric cases. We confirmed some studies that found RTX able to improve patients’ BMD [17, 20, 21]. However, the T-score of BMD failed to show any significant difference. Each diagnostic model had certain limitations, and better diagnostic criteria are required. Moreover, the heterogeneity we found in some studies prevented us from conducting a more robust analysis.
RTX reduced autoantibody levels [25] and ameliorated chronic inflammatory diseases mediated by T and B cells [26]. Kamburova et al. [27] found that RTX induces stronger T-cell proliferation (especially Th2-like cells) by B cell stimulation when compared to untreated patients. This partially explains our finding that the Th1/Th2 ratio was lower after RTX administration.
RTX depletes B cells through antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and apoptosis [28, 29], which determine the therapeutic use in malignant B-cell lymphoma [30], and the reduced auto-antibodies in various autoimmune disorders [31, 32]. Moreover, B cells had an additional role in producing permeability factors with T cells, which provided a rationale for RTX therapy [33]. Interestingly, Fornoni et al. [34] found that RTX prevents the disruption of podocyte apoptosis and actin cytoskeleton through the phosphodiesterase acid-like 3b. All of these data along with our results demonstrate the efficacy of RTX in the treatment of SDNS.
There are some limitations of our analysis. First, the studies included were not randomized controlled trials. Kamburova et al. [35] demonstrated the effect of RTX on the immune response not only through B cell depletion, but also through the cellular functions of the remaining B cells. Thus, the efficacy of RTX might not be as optimistic as we observed. Second, compared with baseline condition, it whittled down self-healing capacity, but it cab eliminate individual difference at the extreme. Third, Munyentwali et al. [15] observed that the relapse of steroid-dependent minimal change disease usually occurred after the reappearance of CD19 cells. Unfortunately, there are no further analyses of the temporal relations between relapse times and CD19 cell count.
In conclusion, so far, RTX has proved to be an effective and well-tolerated drug for the treatment of SDNS. However, more studies are needed to better evaluate its efficacy, long-term safety and mechanism of action.