Frequently relapsing (FR) nephrotic syndrome (NS)/steroid-dependent (SD) NS (FRSDNS) has frequent relapses and require repeated or ongoing use of glucocorticoids. Children who had a relapse at least twice within half a year or three times within 1 year were defined as FRNS. Patients who had a relapse on a reducing course of prednisolone or within 2 weeks of stopping steroids were diagnosed with SDNS. Relapses in patients with FRSDNS are closely related to infections, thrombosis, and dyslipidemia [1, 2]. Immunosuppressive drugs, e.g., cyclosporine A (CyA), mycophenolate mofetil (MMF), cyclophosphamide, and tacrolimus are efficacious in FRSDNS [1–3]. A meta-analysis of randomized controlled trials (RCTs) reported cyclophosphamide to be more effective than CyA and preferred initially in children with FRSDNS [2]. Although tacrolimus is often associated with nephrotoxicity, it could induce remission in CyA and MMF resistant pediatric onset of NS [4].
Since 2004, several studies [5–7] have reported that rituximab, a chimeric anti-CD20 monoclonal antibody maintains remission in complicated FRSDNS. Sinha et al. [5] showed that the median time to first relapse with single-dose of rituximab (375 mg/m2) in children with SDNS and 2–4 doses were 16 and 18 months, respectively. More FRSDNS children achieved complete withdrawal of steroids within 3 months with rituximab as the first steroid-sparing agent than cyclophosphamide [7]. A comprehensive search of PubMed for relevant papers published before July 2021 found only three RCTs that documented a higher 1-year relapse-free survival rate and lower cumulative corticosteroid dose with rituximab than conventional drugs, MMF, and tacrolimus as the first steroid-sparing agent in children with SDNS [3, 8, 9].
Materials
A prospective randomized study of 51 children with FRSDNS not previously treated with steroid-sparing agents were randomized to cyclophosphamide, tacrolimus, or rituximab. Patients completed 6–8 doses of IV cyclophosphamide (10 mg/kg/day for 2 days, biweekly; maximum cumulative dose: 168 mg/kg), along with intravenous 3500 ml/m2 per 24 h one-third saline hydration and bicarbonate (2–3 ml/kg) alkalization. Children received tacrolimus, 0.1–0.15 mg/kg per day in 2 divided doses for 6 months, ensuring a tacrolimus blood concentration of 4–8 ng/ml. The tacrolimus dosage was gradually reduced and eventually stopped within 1.5–2 years.
Children were scheduled to receive a single-dose of rituximab (375 mg/m2 body surface area; maximum dose, 500 mg). They also received a prophylactic dose of trimethoprim (3 mg/kg per time, QOD) for 3 months, which was continued for 6 months in cases where the CD4+ T-cell counts were below 410 cells/μl. At 5 and 30 min before rituximab infusion, children were administered dexamethasone (0.2 mg/kg, intravenous injection) and promethazine (0.125 mg/kg, intramuscular injection). Rituximab was re-administered (375 mg/m2; if CD19+ B-cell counts < 90 cells/μl, half-dose of 375 mg/m2) at 6 months’ intervals. Prednisolone was stopped by 3 months for all groups. Patients who experienced a relapse following their allocated treatment received remission-induction steroids again. Prednisolone was re-administered at a dose of 2 mg/kg/day for 1–2 weeks, then 2 mg/kg/day every other day for 2–3 weeks, then tapered by 0.25 mg/kg every 2 weeks, and stopped within 3 months.
Statistical analysis
Statistical analyses were carried out using SPSS 19.0. Continuous data are expressed as mean (standard deviation). Data at different time-points or among three groups were analyzed using paired t-test or one-way ANOVA. Adverse events were compared using the χ2 test. Kaplan-Meier analysis and Cox regression model were used to analyze and compare relapse-free survival. Pearson’s test was used to assess pre-treatment HRQOL and NS duration. Statistical significance was set at p < 0.05.
Results
The baseline demographic and clinical characteristics were well balanced among the treatment groups. Our study demonstrated a significant and clinically relevant reduction in the mean relapse rate during the 6 months after treatment with rituximab, tacrolimus, and cyclophosphamide compared to 6 months before treatment. The mean prednisolone dosage was significantly lower in all groups, confirming that these drugs are effective in minimizing relapse frequency in children with FRSDNS [1, 2, 7, 8].
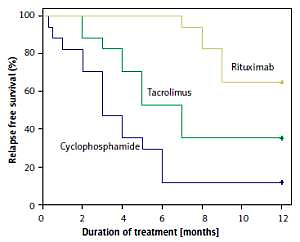
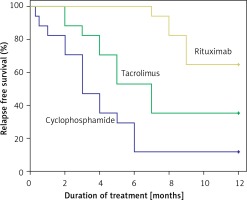
The 1-year relapse-free survival rate was significantly higher in patients treated with rituximab (82.4%) than in those treated with tacrolimus (64.7%, p = 0.043) and cyclophosphamide (11.8%, p < 0.001) (Figure 1). In patients who relapsed in the first year, the mean time to first relapse was longest with rituximab (8.3 months), followed by tacrolimus (4.6 months) and cyclophosphamide (3.3 months) (p < 0.001). An RCT by Basu et al. [8] reported a longer median time to first relapse with rituximab (40 weeks) than with tacrolimus (29 weeks). Another non-RCT showed a higher 1-year relapse-free survival with rituximab (84.2%) than cyclophosphamide (58.6%) [7]. Therefore, rituximab was more effective than cyclophosphamide and tacrolimus in prolonging the remission time.
Figure. 1
Relapse-free survival during 1-year follow-up comparing patients treated with rituximab, tacrolimus, and cyclophosphamide. The 1-year relapse-free survival was 82.4% with rituximab vs. 64.7% with tacrolimus (adjusted HR = 2.346; 95% CI: 0.586–9.389; p = 0.228) vs. 11.8% with cyclophosphamide (adjusted HR = 5.082; 95% CI: 1.415–18.253; p = 0.013)
A key aim of steroid-sparing agents is to minimize the use of glucocorticoids [7, 8]. Concurring with previous observations, within 1 year after therapy, the rituximab group had the lowest mean cumulative prednisolone dose (p = 0.016) and mean relapse rate (p = 0.030) (Table I). At 12 months, 14 children with rituximab, 10 with tacrolimus, and 9 with cyclophosphamide did not receive prednisolone; children receiving rituximab had higher serum albumin and lower cholesterol levels than those treated with cyclophosphamide and tacrolimus (p < 0.05). While all groups showed increased estimated glomerular filtration rate (eGFR) levels compared to pre-treatment, the final eGFR was highest in the rituximab group (p < 0.05). The rituximab group also had the lowest relapse rate. These results validate rituximab’s better efficacy in alleviating prednisolone usage and relapse rate compared to the other drugs. It also lowers serum cholesterol levels, increases eGFR and serum albumin levels [7, 8].
Table I
Relapses, infections, and the cumulative prednisolone dosage in FRSDNS patients after 1 year of treatment with cyclophosphamide, tacrolimus, or rituximab
| Treatment | N | Relapse | Infection | Cumulative prednisolone dosage [mg/kg] |
|---|---|---|---|---|
| Cyclophosphamide | 17 | 1.2 (0.6) | 2.6 (1.3) | 119.2 (58.4) |
| Tacrolimus | 17 | 1.1 (0.9) | 1.6 (1.0)a | 101.7 (72.5) |
| Rituximab | 17 | 0.5 (0.6)ab | 1.1 (0.7)a | 53.2 (33.2)ab |
| F | 3.780 | 8.420 | 4.611 | |
| P-value | 0.030 | 0.001 | 0.016 |
Discussion
In this study, we used repeated rituximab or oral prednisolone as maintenance therapies. Consistently with our findings, previous studies have reported comparable relapse-free survival with rituximab (375 mg/m2) followed by maintenance therapy (750 mg/m2), and (1125–1500 mg/m2) in children with FRSDNS [10]. In another study, 13 SDNS patients discontinued all drugs and maintained complete remission for 25 months after receiving repeated rituximab following single-dose of rituximab [11]. However, another study showed no change in the relapse-free interval in 58 children with FRSDNS despite 2–7 repeated courses of rituximab [12]. Although repeated courses of rituximab appear to be safe, we found that some children developed hypogammaglobulinemia. Therefore, the long-term effects of repeated use of rituximab remain to be evaluated.
Interestingly, in our study, 15 of 17 participants with more infections and presumably infection-related relapses within 12 months used cyclophosphamide, although our regimen and total dose were similar to those previously reported [1, 7]. Data from RCTs have shown that cyclophosphamide reduces the number of relapses by about half in 6–12 months compared to prednisolone [1]. However, Kari et al. [7] have suggested that approximately 50% of the patients remained in remission, which is inconsistent with our findings. The reasons for cyclophosphamide-related infections include inhibition of the immune function, particularly humoral immunity. Short-term high-dose usage of cyclophosphamide could further lower children’s immunity.
Within 1 year, 108 adverse events were recorded: 24 in the rituximab, 34 in the tacrolimus, and 50 in the cyclophosphamide groups (p = 0.327). The difference was mainly accounted for by infections, which occurred nearly twice as often with cyclophosphamide than with rituximab and tacrolimus (p = 0.001) (Table I). Most patients relapsed along with infection before completion of cyclophosphamide therapy (Figure 1).
However, data directly comparing the health-related quality of life (HRQOL) in children with FRSDNS using steroid-sparing agents are scarce [6, 13–15]. In a study of 78 “difficult-to-treat” NS patients, Roussel et al. [13] showed that HRQOL scores for patients in stable remission, rituximab-treated, and treated with other drugs were 74.7, 72.6, and 76.2, respectively. “School functioning” scores during stable remission were lower in rituximab-treated patients. A recent study demonstrated that the global quality of life scores improved 6 months after rituximab infusion compared to pre-treatment, especially the physical, emotional, and school functioning scores [6].
HRQOL was evaluated before and 1 year after treatment with these drugs using PedsQL™ 4.0 Generic Core Scales [14, 15]. Patients aged 2–4 years (n = 11), 5–7 years (n = 19), 8–12 years (n = 17), and 13–18 years (n = 4) completed the PedsQL™ questionnaire. The NS duration showed a negative correlation with the pre-treatment total health score (p = 0.886). Before treatment, the three groups had similar total health scores in each domain (p > 0.05).
In conclusion, our study showed improved psychological health summary and social and school functioning scores 1 year after rituximab treatment, compared to pre-treatment. Better total HRQOL scores, especially related to social and school functioning, have been reported for patients with incident NS than prevalent NS [14]. Another study on 21 NS patients found that the total HRQOL scores improved by 10 points or more over a 2-year rituximab treatment, administered four times at 6-month intervals [15].
At 1 year after treatment, the rituximab group had higher total, psychological health summary, and social and school functioning scores than the cyclophosphamide and tacrolimus groups, indicating a higher HRQOL with rituximab. In contrast, compared to children with NS who received no medication, those on other steroid-sparing agents (tacrolimus, CyA, oral cyclophosphamide, MMF) for 6 months showed lower overall HRQOL scores [14]. Additionally, no association was seen between the HRQOL scores and functioning domains (physical, emotional, social, and educational), consistent with our findings that cyclophosphamide and tacrolimus did not affect the functioning domain scores [14].