Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
DERMATOLOGY / RESEARCH PAPER
Serum γ-Klotho and Fibroblast Growth Factor-2 Levels in Patients with Pressure Ulcers
1
Sultangazi Haseki Training and Research Hospital, Turkey
2
Health Science University, Kartal Dr. Lutfi Kırdar City Hospital, Turkey
Submission date: 2025-05-28
Final revision date: 2025-07-09
Acceptance date: 2025-08-03
Online publication date: 2025-10-05
Corresponding author
KEYWORDS
TOPICS
ABSTRACT
Introduction:
This study aimed to evaluate the serum levels of γ-Klotho and fibroblast growth factor 2 (FGF2) in patients with pressure ulcers.
Material and methods:
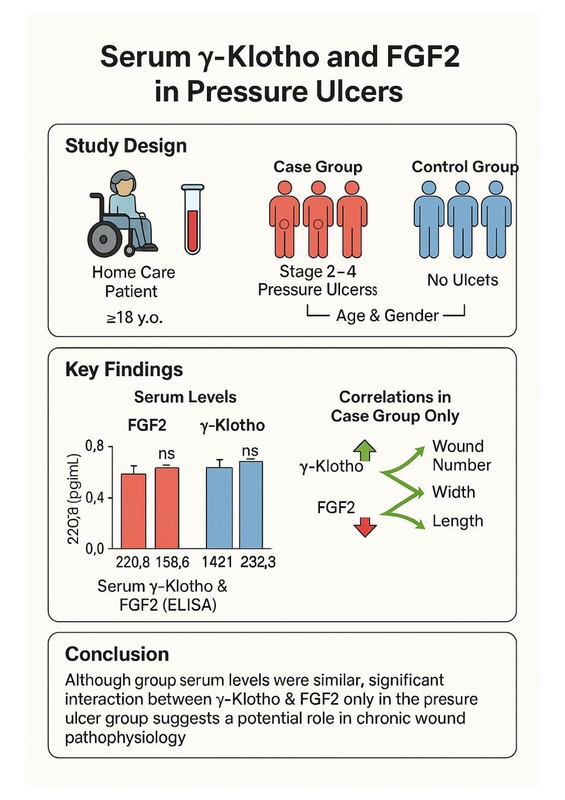
Patients aged ≥18 years receiving care from Home Health Services were included. The case group consisted of individuals with stage 2–4 pressure ulcers, while the control group comprised patients without ulcers, matched for age or gender. Sociodemographic characteristics, medical device usage, and nutritional status were assessed for all participants. In the case group, additional data regarding ulcer site, stage, number, and dimensions were collected. Serum levels of γ-Klotho and FGF2 were measured using the ELISA method.
Results:
A total of 80 participants were enrolled (40 (50.0%) in each group). FGF2 level was 220.8 [26.8] pg/mL in the case group and 219.1 [29.1] pg/mL in the control group (p=0.577). Similarly, γ-Klotho was 1448.4 [158.6] pg/mL in the case group and 1421.3 [232.8] pg/mL in the control group (p=0.453). Conversely, a significant correlation was observed between γ-Klotho and FGF2 levels in the case group (p = 0.048), whereas no such association was found in the control group (p = 0.164). Additionally, FGF2 levels were positively associated with the number, width, and length of the wounds (p=0.003, p=0.007, and p=0.012, respectively), while a negative correlation was observed between γ-Klotho levels and wound duration (p=0.026).
Conclusions:
Although serum levels did not differ between groups, the exclusive correlation between γ-Klotho and FGF2 in the pressure ulcer group may indicate a role in chronic wound processes, warranting further investigation.
This study aimed to evaluate the serum levels of γ-Klotho and fibroblast growth factor 2 (FGF2) in patients with pressure ulcers.
Material and methods:
Patients aged ≥18 years receiving care from Home Health Services were included. The case group consisted of individuals with stage 2–4 pressure ulcers, while the control group comprised patients without ulcers, matched for age or gender. Sociodemographic characteristics, medical device usage, and nutritional status were assessed for all participants. In the case group, additional data regarding ulcer site, stage, number, and dimensions were collected. Serum levels of γ-Klotho and FGF2 were measured using the ELISA method.
Results:
A total of 80 participants were enrolled (40 (50.0%) in each group). FGF2 level was 220.8 [26.8] pg/mL in the case group and 219.1 [29.1] pg/mL in the control group (p=0.577). Similarly, γ-Klotho was 1448.4 [158.6] pg/mL in the case group and 1421.3 [232.8] pg/mL in the control group (p=0.453). Conversely, a significant correlation was observed between γ-Klotho and FGF2 levels in the case group (p = 0.048), whereas no such association was found in the control group (p = 0.164). Additionally, FGF2 levels were positively associated with the number, width, and length of the wounds (p=0.003, p=0.007, and p=0.012, respectively), while a negative correlation was observed between γ-Klotho levels and wound duration (p=0.026).
Conclusions:
Although serum levels did not differ between groups, the exclusive correlation between γ-Klotho and FGF2 in the pressure ulcer group may indicate a role in chronic wound processes, warranting further investigation.
REFERENCES (18)
1.
Anders J, Heinemann A, Leffmann C, Leutenegger M, Pröfener F, von Renteln-Kruse W. Decubitus Ulcers: Pathophysiology and Primary Prevention. Dtsch Arztebl Int 2010;107(21):371–382.
2.
Yamauchi M, Hirohashi Y, Torigoe T, et al. Wound healing delays in a-Klotho-deficient mice that have skin appearance similar to that in aged humans e Study of delayed wound healing mechanism. Biochem Biophys Res Commun 2016;473(4):845-852.
3.
Laiva AL, O’Brien FJ, Keogh MB. Anti-Aging-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells. Pharmaceuticals (Basel) 2021;14(11):1168.
4.
Koike Y, Yozaki M, Utani A, Murota H. Fibroblast growth factor 2 accelerates the epithelial–mesenchymal transition in keratinocytes during wound healing process. Sci Rep 2020;10(1):18545.
5.
Liu Y, Liu Y, Deng J, Li W, Xuqiang N. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front Endocrinol (Lausanne) 2021;12:744868.
6.
Farooq M, Khan AW, Kim MS, Choi S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021;10(11):3242.
7.
Ornitz DM, Itoh N. New developments in the biology of fibroblast growth factors. WIREs Mech Dis 2022;14(4):e1549.
8.
Petit I, Levy A, Estrach S, et al. Fibroblast growth factor‑2 bound to specific dermal fibroblast‑derived extracellular vesicles is protected from degradation. Sci Rep 2022;12(1):22131.
9.
Syromiatnikova VY, Kvon AI, Starostina IG, Gomzikova MO. Strategies to enhance the efficacy of FGF2-based therapies for skin wound healing. Arch Dermatol Res 2024;316(7):405.
10.
Zhang J, Liu Z, Li Y, et al. FGF2: a key regulator augmenting tendon-to-bone healing and cartilage repair. Regen Med 2020;15(9):2129-2142.
11.
Sun F, Liang P, Wang B, Liu W. The fibroblast growth factor–Klotho axis at molecular level. Open Life Sci 2023;18(1):20220655.
12.
Hajare AD, Dagar N, Gaikwad AB. Klotho antiaging protein: molecular mechanisms and therapeutic potential in diseases. Mol Biomed 2025;6(1):19.
13.
Scazzone C, Agnello L, runa Lo Sasso BL, et al. Klotho and vitamin D in multiple sclerosis: an Italian study. Arch Med Sci 2020;16(4):842–847.
14.
Liu J, Shi D, Song X, Ji Y. Soluble α-klotho as a potential predictor of all-cause mortality in Chinese maintenance hemodialysis patients. Arch Med Sci. (in press).
15.
Tyurenkov IN, Perfilova VN, Nesterova AA, Glinka Y. Klotho Protein and CardioVascular System. Biochemistry (Mosc) 2021;86(2):132-145.
16.
El-Saeed WS, Elnagdy MH, Abd elghaffar MA, Elbaz A, Zahran MA. Role of alpha and gamma Klotho genes in the development of differentiated thyroid carcinoma on top of goiter. FCO 2023;13(3):31–39.
17.
Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, Aging, and the Failing Kidney. Front Endocrinol (Lausanne) 2020;11:560.
18.
Li X, Wang C, Xiao J, McKeehan WL, Wang F. Fibroblast growth factors, old kids on the new block. Semin Cell Dev Biol 2016;53:155-167.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.