Introduction
Anaesthetics are used to manage pain during surgery and medical procedures in children, infants, and adults [1]. However, anaesthetics affect learning and memory in the developing brain by inducing neuronal apoptosis [2]. The brain cells of neonatal rats were damaged more by isoflurane than by an equal dose of sevoflurane [3]. Isoflurane enhances neuronal cell apoptosis by altering the mitochondrial membrane [4]. The Bcl-2/Bax ratio plays an important role in maintaining the integrity of the mitochondrial membrane, which controls neuronal apoptosis [5].
In a murine model, isoflurane exposure enhanced the expression of caspase-3 and deposition of β-amyloid peptide (Aβ), which contributes to the cellular apoptotic pathway [6]. Anaesthetics enhance neurodegeneration by increasing the levels of pro-inflammatory cytokines [7] such as interleukin (IL) and tumor necrosis factor α (TNF-α), which contribute to the nuclear factor-κB (NF-κB)-dependent signalling pathway, leading to cognitive impairment [8]. In cerebral ischaemia, the TLR-4 pathway is regulated by NF-κB, and a reduction in TLR-4 expression downregulates inflammation in ischaemic stroke [9]. Therefore, ischaemia might be managed by targeting the TLR-4 pathway.
Alternative medicine has gained importance in the management of several chronic disorders, including neuronal injury. Sophora japonica (Leguminosae) is used as a traditional medicine in China, and sophoricoside has been isolated from it [10]. Sophoricoside has anti-hyperglycaemic, anti-obesity, antioxidant, anticancer [11], anti-inflammatory, and anti-allergic properties, as it reduces cycloxygenase-2 activity and IL-6 levels [12]. Therefore, this study evaluated the protective effects of sophoricoside on cognitive dysfunction in isoflurane-induced neuronal injury rats.
Material and methods
Animals
Sprague-Dawley rat pups at P1 were housed under standard conditions with a 12-h light/dark cycle at 25 ±3°C and 60 ±5% humidity. All animal procedures were approved by the Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University, China (IAEC/FAH-ZU/2018/02).
Chemicals
Sophoricoside and isoflurane were procured from Sigma Aldrich, USA. Enzyme-linked immunosorbent assay (ELISA) kits for macrophage inflammatory protein (MIP)-1α, MIP-1β, and the inflammatory mediators IL-1β, IL-6, and TNF-α were purchased from R&D Systems, USA. Antibodies used in the Western blot assay were bought from Thermo Fisher Scientific, USA.
Experimental
In a controlled chamber, pups were exposed to isoflurane (0.75%) with 30% oxygen for 6 h on P7. The pups were separated into normal, isoflurane, and sophoricoside 8 and 16 groups (n = 12 each). The sophoricoside 8 and 16 groups were given 8 and 16 mg/kg sophoricoside, respectively, for 21 days after isoflurane exposure. At the end of the experiment, the animals were sacrificed, and the hippocampus was removed. Isolated samples of brain were kept at –80ºC until required.
Morris water maze test
Cognitive function was estimated at the end of the treatment using the Morris water maze (MWM) [13]. Spatial memory was assessed by conducting trials on 5 consecutive days and recording the time spent in the target quadrant on the last day, after the platform was removed. The DigBehav System was used to record and analyse the data.
Neurological dysfunction
Neurological dysfunction was assessed using Longa’s method [14]. The scoring was as follows: 0, no deficit; 1, forelimb failed to extend entirely and weak; 2, circling to the contralateral side; 3, weight-bearing capacity reduced on the injured side; and 4, no spontaneous locomotor activity.
TUNEL assay
To evaluate neuronal apoptosis, TUNEL assays were performed as previously reported [15]. The hippocampus was sectioned in 5-µm-thick slices, and the DeadEnd fluorometric TUNEL System kit was used to estimate neuronal apoptosis. Nuclei of the neurons were stained with Hoechst, and NIS-Elements Basic Research (BR) imaging software was used to determine the number of TUNEL-positive cells.
Estimation of cytokines
The levels of MIP-1α, MIP-1β, IL-1β, IL-6, and TNF-α were measured in brain tissue homogenates using ELISA as per the kit protocols.
Western blotting
The expression of TLR-4, NF-κB, p-Akt, Akt, p-PI3K, and PI3K proteins was assessed using Western blot assays of brain tissue homogenates. A BCA assay kit was used to quantify the protein from each tissue homogenate, and 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis was used to separate the proteins, which were transferred to nitrocellulose membranes by electroblotting. Subsequently, each membrane was blocked with 5% blocking solution (non-fat milk) and incubated in blocking buffer with primary antibodies overnight at 4°C. Goat secondary antibodies conjugated with horseradish peroxidase were added to the blocking buffer, and a chemiluminescence kit was used to detect the proteins.
qRT-PCR
TRIzol reagent was used to extract the total RNA, and TaqMan MicroRNA assays were used estimate the mRNAs levels. Moloney murine leukaemia virus reverse-transcriptase was used to synthesise complementary DNA from 2 µg of total RNA in a reverse transcriptase reaction (20 µl). The primers listed below were mixed with RT 2 SYBR Green Master Mix to determine gene expression using the Quantitative SYBR Green PCR assay. The relative target gene expression levels were determined using the 2–ΔΔCq equation (Table I).
Histopathological analysis
Formaldehyde (4%) was used to fix the brain tissues, which were embedded in paraffin, sectioned to 5-µm thicknesses using a microtome, and stained with haematoxylin and eosin (H&E). A trinocular microscope was used to evaluate pathophysiological changes in the brain tissue.
Statistical analysis
All data are expressed as the mean ± SEM (n = 12). The statistical analysis was performed using one-way analysis of variance (ANOVA). Post hoc comparison of means was performed using Dunnett’s post hoc test ver. 4.1 (GraphPad Software, San Diego, CA, USA). The level of statistical significance was set at p < 0.05.
Results
Sophoricoside alleviates cognitive dysfunction
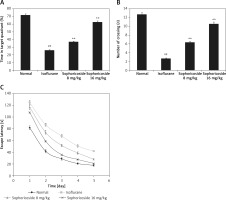
Cognitive function was assessed in sophoricoside- and isoflurane-treated rats using the MWM, as shown in Figure 1. Greater decreases were found in the time spent in the target quadrant, number of crossings, and escape latency in the isoflurane-treated group than in the controls. Sophoricoside treatment increased the time spent in the target quadrant, number of crossings, and escape latency compared with the isoflurane-induced neuronal injury rats.
Sophoricoside alleviates neurological dysfunction
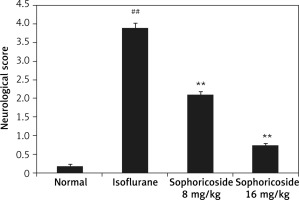
Neurological dysfunction was assessed by determining the neurological score in anaesthesia-induced neuronal injury rats (Figure 2). The neurological score was significantly (p < 0.01) higher in the isoflurane-treated group than in the normal rats. A dose-dependent reduction was observed in the sophoricoside-treated groups compared to the isoflurane-treated group.
Sophoricoside alleviates neuronal apoptosis
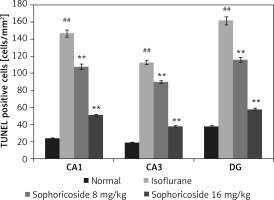
Figure 3 shows neuronal apoptosis in the hippocampus of isoflurane- and sophoricoside-treated rats using the TUNEL assay. The number of apoptotic neuronal cells was determined by assessing the number of TUNEL-positive cells in the hippocampus tissue. This value was lower in the hippocampus tissue (CA1, CA3, and DG) of the isoflurane group than in the normal rats. However, sophoricoside treatment reduced neuronal apoptosis in the isoflurane-induced neuronal injury rats.
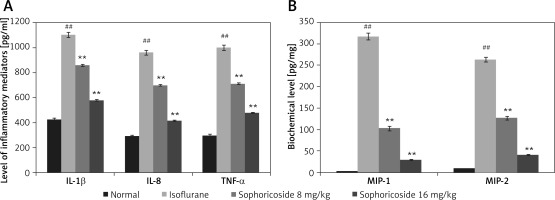
Sophoricoside reduces cytokine and macrophage inflammatory protein levels
In the brain tissues of the isoflurane- and sophoricoside-treated groups, the levels of macrophage inflammatory proteins and inflammatory cytokines were assessed, as shown in Figure 4. Significant increases in the levels of MIP-1, MIP-2, TNF-α, IL-8, and IL-1β were observed in the hippocampus tissues of the isoflurane group compared with the normal group. The levels of cytokines and macrophage inflammatory proteins were attenuated in the brain tissues of the sophoricoside-treated groups compared with the isoflurane group.
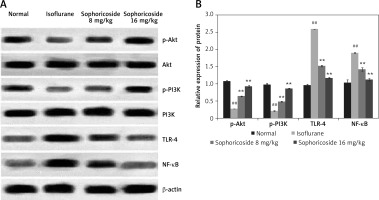
Sophoricoside alleviates the altered expression of NF-κB, TLR-4, Akt, and PI3K proteins
The expression of NF-κB, TLR-4, Akt, and PI3K proteins was determined in the brain tissues of isoflurane- and sophoricoside-treated rats by Western blot assays. A significant reduction in the expression of p-Akt and p-PI3K was observed in the brain tissues of the isoflurane group compared to the normal group, whereas the expression of NF-κB and TLR-4 was enhanced in the isoflurane group. Sophoricoside treatment normalised the altered expression of NF-kB, TLR-4, Akt, and PI3K proteins in the brain tissues of isoflurane-induced neuronal injury rats (Figure 5).
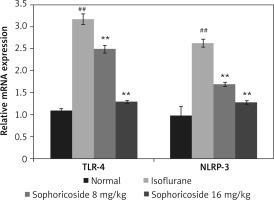
Sophoricoside alleviates altered TLR-4 and NLRP3 mRNA expression
The effect of sophoricoside on NLRP-3 and TLR-4 mRNA expression in the brain tissues of neuronal injury rats was assessed by RT-PCR (Figure 6). The expression of NLRP-3 and TLR-4 mRNA was enhanced in the hippocampus tissue of the isoflurane group compared to the normal rats. The NLRP-3 and TLR-4 mRNA expression was reduced in the brain tissue of the sophoricoside-treated groups compared to the isoflurane group.
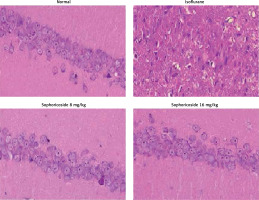
Sophoricoside alleviates histopathological changes
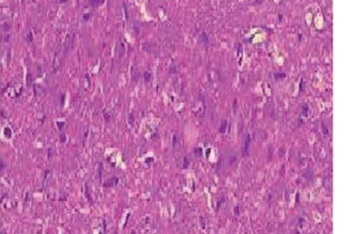
Figure 7 shows histopathological changes in the brain tissue of isoflurane-induced neuronal injury rats with H&E staining. Brain tissue sections of the control group appeared normal. The brain tissues of the isoflurane group showed nuclear and cytoplasmic vacuolation in the white matter and cortex. Moreover, the neurocytes were unconsolidated and disordered. However, sophoricoside treatment ameliorated these pathological changes in the brain tissues of isoflurane-induced neuronal injury rats.
Discussion
The administration of anaesthetics to children and neonates leads to neuronal injury, which causes memory impairment and loss of cognitive function [16]. This study examined the protective effect of sophoricoside in isoflurane-induced neuronal injury rats by assessing cognitive function using the neurological score and MWM. Neuronal apoptosis was assessed in hippocampus tissue using a TUNEL assay, and the levels of cytokines and macrophage inflammatory proteins were assessed by ELISA. Western blotting and RT-PCR were performed to assess the expression of NF-κB, TLR-4, Akt, and PI3K proteins in neuronal tissues. Immunohistochemical and histopathological changes were observed in the brain tissue of isoflurane-induced neuronal injury rats.
Anaesthetics are used commonly and can cause neuronal damage in the developing brain, resulting in loss of memory and cognitive function [17]. In animals exposed to isoflurane, neuronal cells are damaged via activation of the apoptosis pathway [18], as supported by our data. However, sophoricoside treatment reversed the altered cognitive function and reduced neuronal apoptosis in isoflurane-exposed neuronal injury rats.
The Akt/PI3K pathway is responsible for cell stability, growth, and survival. Anti-apoptotic protein activation and the deactivation of pro-apoptotic factors are regulated by the Akt/PI3K pathway [19]. The developing brain exposed to isoflurane shows altered expression of PI3K and Akt protein, as reported previously [20], and attenuation of the Akt/PI3K pathway protects against neuronal injury [21]. Our data suggest that sophoricoside treatment regulates the Akt/PI3K pathway in the neuronal tissues of neuronal injury rats.
Mediators of inflammation are also involved in cognitive defects and neuronal injury observed in developing brains exposed to isoflurane [22, 23]. These mediators enhance the inflammatory process by activating NF-κB signalling. We found a significant (p < 0.01) reduction in inflammatory cytokines in the brain tissue of the sophoricoside-treated groups compared to the isoflurane group. TLR4 is a transmembrane protein that regulates innate immunity by activating inflammatory cytokines and NF-κB signalling. TLR-4 activation contributes to neuronal injury by regulating pro- and anti-apoptotic proteins [24], and controlling TLR-4 signalling prevents neuronal apoptosis [25]. Our data suggest that sophoricoside treatment ameliorates altered TLR-4 signalling in a neuronal injury rat model.
In conclusion, sophoricoside treatment protects against neuronal injury and preserves cognitive function in isoflurane-induced neuronal injury rats by regulating TLR-4 signalling. Moreover, it controls neuronal apoptosis by regulating inflammatory cytokines.