Almost 7 years ago, the 2018 ADA/EASD guidelines shifted the management of type 2 diabetes mellitus (T2DM) from a “glucocentric” to a “cardiorenal” approach based on the increased cardiovascular (CV) and renal risk of these patients and the important benefits in terms of cardiorenal morbidity and mortality of certain antidiabetic drugs, namely sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) [1, 2]. This therapeutic strategy highlights the involvement of both cardiac and kidney dysfunction in the pathophysiology of T2DM and its complications, and it is further supported by the recent ADA/EASD guidelines [3, 4]. Furthermore, the implication of liver dysfunction in the development and outcomes of T2DM has also been progressively recognized [5, 6].
Herein, we discuss the concept of regarding T2DM as a Cardiac, Kidney and Liver (CKL) syndrome.
T2DM and cardiac dysfunction
Traditionally, T2DM has been associated with macrovascular complications, including coronary heart disease (CHD), stroke, carotid and peripheral artery disease [7–11]. According to a systematic review of 4,549,481 T2DM patients, CHD was the most frequently reported form of CV disease (21.2%) [12]. Apart from acute coronary syndrome (ACS) incidence, T2DM patients also have an increased risk of ACS mortality [13, 14]. Of note, in a previous meta-analysis (30 prospective cohort studies, n = 1,148,188 individuals), the pooled women-to-men relative risk ratio (RRR) was 1.52 (95% CI: 1.32–1.76; p < 0.001) for CHD, 1.23 (95% CI: 1.09–1.39; p = 0.001) for stroke, 1.49 (95% CI: 1.11–2.00; p = 0.009) for cardiac death and 1.51 (95% CI: 1.23–1.85; p < 0.001) for total mortality [15]. These findings suggest that T2DM women are at a greater risk for CV morbidity and mortality, as well as all-cause death compared with T2DM men.
T2DM has also been linked to arrythmias (e.g., atrial fibrillation (AF)) [16, 17] and sudden cardiac death (SCD) [18]. Regarding AF, a previous meta-analysis including 8 cross-sectional studies (n = 39,898 participants) showed that patients with diabetes had a 1.31-times higher likelihood of having non-paroxysmal rather than paroxysmal AF (pooled OR = 1.31, 95% CI: 1.13–1.51, I2 = 82.6%) [19]. Furthermore, another meta-analysis found that both prediabetes (RR = 1.20, 95% CI: 1.03–1.39, I2 = 30%; n = 42,392 cases, 58,547 participants) and diabetes (RR = 1.28, 95% CI: 1.22–1.35, I2 = 90%; n = 31,249,772 cases, 10,244,043 participants) were linked to an increased risk for AF [20]. In terms of SCD, another meta-analysis, involving 19 population-based prospective studies (3,610 cases, 249,225 participants), the adjusted relative risk (RR) for SCD was 2.02 (95% CI: 1.81–2.25, I2 = 0%) for diabetes patients vs. non-diabetics and 1.23 (95% CI: 1.05–1.44, I2 = 6%) for patients with pre-diabetes vs. non-diabetics [21]. The same meta-analysis also analyzed 10 patient-based prospective studies (2,713 cases, 55,098 participants) and found that the adjusted RR was 1.75 (95% CI: 1.51–2.03, I2 = 39%) for diabetes patients vs. non-diabetics, 1.63 (95% CI: 1.36–-1.97, I2 = 39%) for CHD patients and 1.85 (95% CI: 1.48–2.33, I2 = 0%) for HF patients, separately [21].
T2DM has been associated with heart failure (HF) development and progression [22]. This has been recognized by the European Society of Cardiology (ESC), the American College of Cardiology (ACC) and the American Heart Association (AHA) in their current guidelines for the management of HF [23, 24] as well as the 2023 ESC guidelines for CV disease (CVD) management in T2DM patients [25]. A previous meta-analysis evaluated the association between diabetes and the risk for new-onset HF (in 74 cohort studies) and recurrent HF (in 38 cohort studies) [26]. For new-onset HF, the pooled RR was 2.14 (95% CI: 1.96–2.34) when HF was regarded independently of ejection fraction (EF), whereas RR was 2.22 (95% CI: 2.02–2.43) in 7 cohort studies that examined separately HF with preserved EF (HFpEF) and 2.73 (95% CI: 2.7–2.75) in 8 studies that evaluated HF with reduced EF (HFrEF) [26]. For recurrent HF, the pooled RR was 1.39 (95% CI: 1.33–1.45) for total HF population, 1.73 (95% CI: 1.32–2.26) for studies including only HFpEF patients and 1.37 (95% CI: 1.24–1.50) for studies including only HFrEF patients [26]. Of note, prediabetes may also be related to an increased risk of HF, as shown in meta-analyses [27, 28].
Overall, T2DM has been regarded as a potential CHD equivalent based on common pathophysiology mechanisms (e.g., oxidative stress, inflammation, endothelial dysfunction, platelet activation), risk factors (e.g., obesity, dyslipidemia, hypertension, aging) and outcomes (e.g., HF, arrythmias, CV death and non-cardiac vascular diseases) [29–31]. Epicardial and pericardial adipose tissue also play a role in the development of T2DM-related CHD, AF and HF [32].
T2DM and kidney dysfunction
Chronic kidney disease (CKD) represents a common diabetic microvascular complication (with a prevalence of approximately 40% in T2DM patients), which can occur even at the diagnosis of T2DM [4]. The definition of CKD involves persistent abnormality in kidney function or structure (e.g., albuminuria ≥ 30 mg/day or estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2) for more than 3 months [33]. Of note, albuminuria can be easily assessed in daily practice by the urinary albumin-to-creatinine ratio (UACR). It is important to determine eGFR category (stage 1-5) and albuminuria category (A1-3) in each individual (based on eGFR and UACR levels) in order to plan the therapeutic approach, monitor and follow-up of each patient [33].
CKD has also been linked to adverse CVD outcomes in T2DM patients. For example, a post-hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, involved 10,251 T2DM participants (of them 3,636 patients (35.9%) had CKD defined either as eGFR > 60 ml/min/1.73 m2 or UACR > 30 mg/g) [34]. Among the 3,636 T2DM patients with CKD, 1,449 (14.3%) had stage 1 CKD, 1,366 (13.5%) stage 2 CKD and 821 (8%) stage 3 CKD, whereas in terms of albuminuria, 2,492 (68.8%) had UACR 30-300 mg/g and 668 (18.4%) had > 300 mg/g [34]. Compared with non-CKD, the presence of CKD was associated with a 97% higher risk for all-cause death (hazard ratio (HR) 1.97; 95% CI: 1.70–2.29; p < 0.0001), 119% higher risk for CV mortality (HR = 2.19; 95% CI: 1.76–2.73; p < 0.0001), 87% higher risk for the primary composite outcome (nonfatal myocardial infarction, nonfatal stroke, and CV death) (HR = 1.87, 95% CI: 1.65–2.1; p < 0.0001), as well as higher risks for nonfatal myocardial infarction (MI) by 62%, nonfatal stroke by 149%, any stroke by 141%, major coronary artery disease events by 56% and fatal or nonfatal congestive HF by 219% [34]. Of note, increased rates of the above primary and secondary outcomes were evident even for patients in CKD Stages 1 and 2, as well as in the presence of albuminuria [34]. CKD may also affect prognosis in T2DM patients with CVD. Indeed, a recent meta-analysis (11 studies – n = 25,440 T2DM patients undergoing percutaneous coronary intervention (PCI)) showed that CKD presence was associated with a significantly higher risk of both early and late all-cause death (risk ratio (RR) = 3.45, 95% CI: 3.07–3.87; p < 0.001 and 2.78, 95% CI: 1.92–4.02; p < 0.001, respectively), CVD mortality (RR = 2.90, 95% CI: 1.99–4.22; p < 0.001) and MI (RR = 1.40, 95% CI: 1.06–1.85; p = 0.02) compared with the absence of CKD [35].
Apart from the presence of CKD, CKD progression has also been related to worse cardiorenal outcomes in T2DM patients. In this context, a sub-analysis of both the Action in Diabetes and Vascular Disease: Preterax Controlled Evaluation (ADVANCE) trial and the ADVANCE Post-Trial Observational Study (ADVANCE-ON), involving 8,879 T2DM patients with a mean eGFR of 75 ml/min/1.73 m2, evaluated the associations between annual decline in eGFR slope and the primary outcome (defined as a composite of major cardiorenal events and all-cause mortality) [36]. Over a median follow-up of 7.6 years, an annual substantial decrease in eGFR (i.e., < –1.63 ml/min/1.73 m2/year) significantly correlated with a 30% increased risk for the primary outcome (HR = 1.30, 95% CI: 1.17–1.43; p < 0.001) compared with a stable change in eGFR (defined as –1.63 to 0.33 ml/min/1.73 m2/year) [36]. Similar findings were reported for the secondary outcomes, i.e., 286% increased risk for major renal events (HR = 3.86, 95% CI: 2.55–5.85; p < 0.001), 26% higher risk for major CV events (HR = 1.26, 95% CI: 1.11–1.42; p < 0.001) and 38% increased risk for all-cause mortality (HR = 1.38, 95% CI: 1.22–1.55; p < 0.001) [36]. It should be noted that an annual substantial increase in eGFR (i.e., > 0.33 ml/min/1.73 m2/year) did not significantly affect the outcomes, although a non-significant trend towards reduced rates was observed for both the primary outcomes (HR = 0.96, 95% CI: 0.86–1.07) and the secondary outcomes (HR = 0.59, 95% CI: 0.30–1.16 for major renal events, 0.92, 95% CI: 0.80–1.06 for major CV events and 0.99, 95% CI: 0.86–1.14 for all-cause death) [36].
Similar findings have been reported in a recent prospective cohort study of 6,919 adults (985 with T2DM) followed-up for a median of 8.22 years [37]. Among the T2DM patients, a greater decline in eGFR slope was significantly related to an increased risk of CVD events, even after adjusting for traditional CV risk factors, demographic factors and baseline eGFR; HR for CVD risk was 2.16 (95% CI: 1.09–4.26), 2.39 (95% CI: 1.38–4.12) and 1.76 (95% CI: 1.14–2.70) for eGFR slopes of (−1.05 to –0.74), (−0.74 to –0.67) and (−0.60 to –0.52), respectively, compared with a slope of (−0.51 to –0.16) [37]. In contrast, among individuals without T2DM, the annual eGFR change did not show any significant association with CVD risk [37]. Such findings highlight the potential role of the eGFR slope as a surrogate endpoint for renal outcomes as well as a predictor for all-cause death, CV morbidity and mortality in T2DM patients.
Albuminuria, a marker of systemic endothelial dysfunction [38], may adversely affect CVD risk in T2DM patients, even in the absence of eGFR decline [39]. In this context, in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, 519 T2DM patients had an eGFR < 60 ml/min/1.73 m2 and 2,508 had albuminuria (microalbuminuria defined as > 2.5 mg/mmol for men and >3.5 mg/mmol for women; macroalbuminuria defined as > 25 and > 35 mg/mmol, respectively) [40]. Both micro- and macro-albuminuria were associated with increased CVD risk (HR = 1.25, 95% CI: 1.01–1.54) and 1.19, 95% CI: 0.76–1.85, respectively; p = 0.001 for trend) compared with eGFR ≥ 90 ml/min/1.73 m2 [40]. Of note, in the same study, the HR for eGFR < 60 vs. ≥ 60 ml/min/1.73 m2 was 1.44 (95% CI: 1.18–1.76; p < 0.001) for total CVD risk, 2.00 (95% CI: 1.41–2.84; p < 0.001) for CVD death and 1.66 (95% CI: 1.30–2.11; p < 0.001) for all-cause mortality [40]. Similarly, in a cohort of 742 T2DM patients with CKD followed-up for a median of 4.6 years, the rate of CV events (non-fatal MI, non-fatal stroke, CV death, major amputation and revascularization) increased from 25% in patients with microalbuminuria (defined as 30–300 mg/day) to 33% in those with macroalbuminuria (defined as > 300 mg/day); the corresponding values were 19 to 40% rise in CV risk as eGFR decreased from ≥ 90 to < 45 ml/min/1.73 m2 [41]. Such results highlight the positive association between CKD severity (in terms of both eGFR decline and/or albuminuria progression) and CVD risk in T2DM patients.
A synergistic effect of the presence of albuminuria and a declined eGFR has been described in relation to various causes of mortality in a recent nationwide population-based study involving 2,614,662 T2DM patients [42]. The more advanced the stage of diabetic kidney disease (DKD) was, the higher the incidence rate of death from endocrine and metabolic diseases, as well as genitourinary system disorders, whereas in terms of all-cause and CVD mortality, the highest rate was observed in stage 3 DKD patients [42]. Even in the same eGFR group, patients with albuminuria had a higher risk for mortality due to each cause compared with patients without albuminuria [42]. A similar synergistic effect of albuminuria and decreased eGFR has been described for CKD progression [43]. In particular, among 42,761 T2DM patients from the National Kidney Foundation’s Kidney Early Evaluation Program followed-up for a median of 4 years, those with both eGFR < 30 ml/min/1.73 m2 and macroalbuminuria (defined as UACR > 300 mg/g) had a > 1,000-fold increased risk of progression to end-stage kidney disease than those with eGFR > 60 ml/min/1.73 m2 and UACR < 30 mg/g) [43]. The data supporting a synergistic effect of reduced eGFR and albuminuria on CVD risk in T2DM patients are less evident. In a previous cross-sectional study involving 4,930 insulin-treated T2DM patients, combined presence of reduced eGFR and albuminuria was numerically related to the worst CV outcomes (but this association was significant only for rates of peripheral angioplasty or bypass) [44]. Furthermore, in a sub-analysis of the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial (n = 9,340 T2DM patients at high CV risk), rates of major adverse CV events generally increased with concomitant decreasing baseline eGFR and increasing baseline UACR, but this pattern was not statistically significant, mainly due to the small sample size of relevant subgroups and particularly those at the lowest eGFR levels (< 30 ml/min/1.73 m2), since recruitment was limited by the trial design [45].
Links between T2DM, CKD and HF have also been reported with almost 25–40% of HF patients having T2DM and approximately 40–50% of HF patients having CKD, whereas 16% of HF patients having both T2DM and CKD [46]. The combination of these comorbidities has been related to a substantially increased risk for hospitalization and mortality [46]. Of note, contrast-induced acute kidney injury (CI-AKI) has also been associated with T2DM and CVD risk [47–50], further highlighting the complex interaction between T2DM and the kidneys.
Overall, several pathophysiological mechanisms have been recognized to link T2DM with CKD, including the hyperglycemia-induced advanced glycation end-product formation, oxidative injury, hypoxia, increased production of fibrotic and inflammatory factors, as well as the overactivation of the renin-angiotensin-aldosterone system [51]. Furthermore, endothelial injury, vascular dysfunction, fibrosis and inflammation, apart and beyond hypertension and dyslipidemia, represent some of the major underlying pathways connecting CKD with CVD in T2DM patients (but also in the absence of T2DM) [51].
T2DM and liver dysfunction
There is a growing amount of evidence linking T2DM with liver dysfunction, and especially non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) [52–56]. Indeed, NAFLD may increase by almost 2-fold the risk of developing T2DM, independently of obesity and other common metabolic risk factors [57]. Furthermore, it has been shown that the more advanced the stages of liver fibrosis are, the higher is the risk of T2DM incidence [57]. In this context, a previous meta-analysis (33 studies; n = 501,022 individuals; 27,953 cases of incident T2DM; median follow-up of 5 years) found that NAFLD patients had an increased risk of T2DM incidence compared with those without NAFLD (HR = 2.19, 95% CI: 1.93–2.48) [53]. This risk markedly increased across the severity of liver fibrosis (random-effects HR = 3.42, 95% CI: 2.2–5.11) [53]. Similarly, a recent meta-analysis (156 studies; n = 1,832,125 T2DM patients) found that NAFLD and NASH prevalence rates in T2DM patients were 65.0% (95% CI: 61.8–68.1) and 31.5% (95% CI: 17.1–50.7), respectively [52]. The above results were independent of age, gender, adiposity measures and other metabolic parameters. Indeed, another recent meta-analysis, involving 16 observational studies (304,975 adults with almost 1,300 cases of new-onset T2DM) followed-up for a median of 5 years, reported that the incidence of T2DM was significantly higher in patients with lean NAFLD vs. without NAFLD (HR = 2.72, 95% CI: 1.56–4.74) [58]. Furthermore, HR of incident T2DM in overweight/obese patients without NAFLD and in overweight/obese patients with NAFLD was 1.32 (95% CI: 0.99–1.77) and 2.98 (1.66–5.32) compared with lean patients without NAFLD [58]. Underlying pathophysiological mechanisms include insulin resistance, hyperglycemia, hyperinsulinemia, oxidative stress, inflammation, endothelial dysfunction, dysregulation of adipose tissue function and lipid dysmetabolism (lipotoxicity) [59, 60].
NAFLD/NASH have also been associated with several CV risk factors (such as dyslipidemia, hypertension, obesity, metabolic syndrome), as well as CKD and CVD morbidity and mortality [61–67]. Furthermore, NAFLD patients are at an increased risk of developing HF, especially HFpEF vs. HFrEF [68]. Briefly, in a previous retrospective cohort study including 870,535 Medicare beneficiaries, followed-up for a mean of 14.3 months, NAFLD patients had a significantly higher risk of new-onset HF (adjusted HR = 1.23, 95% CI: 1.18–1.29; p < 0.001), the risk being greater in relation to HFpEF (HR = 1.24, 95% CI: 1.14–1.34; p < 0.001) vs. HFrEF (HR 1.09, 95% CI: 0.98–1.2; p = 0.12) [68]. Of note, a recent meta-analysis including 6 studies with 12,374 HF patients, followed-up for a median of 2.5 years, reported that the presence of NAFLD significantly increased the risk of major adverse outcomes (HR = 1.61, 95% CI: 1.25–2.07), all-cause death (HR = 1.66, 95% CI: 1.39–1.98) and HF (re)hospitalization (HR = 1.71, 95% CI: 1.03–2.86) [69]. These findings highlight the impact of NAFLD, not only on HF development, but also on worse HF prognosis.
Noteworthy, a new nomenclature has been suggested for NAFLD, namely metabolic dysfunction-associated steatotic liver disease (MASLD) [70]. The novel definition aims to highlight the importance of metabolic dysfunction in the pathogenesis of this clinical entity, by also avoiding the stigmatizing term of “fatty liver”, as well as to better categorize different sub-types of MASLD [71, 72]. For example, 2 novel patient groups have been proposed: 1) those with no known cause and no metabolic parameters, characterized to have cryptogenic steatotic liver disease (SLD) and 2) those with MASLD who consume increased amounts of alcohol per week (140–350 g/week and 210–420 g/
week for women and men, respectively), named “metabolic and alcohol related/associated liver disease (MetALD)” [70]. Furthermore, the MASLD definition (i.e., steatosis plus ≥ 1 cardiometabolic risk factor) was, at least in part, driven by a need for a “positive” rather than a “negative” diagnosis, i.e., by excluding excessive alcohol use and other causes of liver disease [73]. Therefore, the new diagnostic criteria have the potential to improve patient identification and disease awareness. Furthermore, MASLD may be able to identify more individuals with high-risk features for progressive liver disease [74]. In this context, a recent meta-analysis including 17 studies (n = 9,808,677 individuals) found that MAFLD was present in 33.0% (95% CI: 29.7–36.5) of the general population, whereas NAFLD in 29.1% (95% CI: 27.1–31.1) [74]. Among FLD patients, 4.0% (95% CI: 2.4–6.4) had NAFLD only and 15.1% (95% CI: 11.5–19.5) had MAFLD only [74]. Interestingly, MASLD was a better predictor of significant fibrosis compared with NAFLD; the MAFLD–only group had a significantly greater risk of liver fibrosis compared with the NAFLD–only group (RR = 4.2, 95% CI: 1.3–12.9) [74]. Similar results have been reported by others [73], as well as that MAFLD may be associated with a higher mortality risk than NAFLD [75]. However, prospective studies (preferably multicenter) are required to define the prevalence and impact of the comorbidities in each disease phenotype on outcomes, thus potentially enabling the selection of specific therapeutical approaches (lifestyle and/or pharmacological) in each MASLD subtype [75].
Apart from NAFLD/MASLD, T2DM has been associated with the presence of excessive adipose tissue depositions in other organs/tissues, such as the heart, vessels, kidneys, pancreas and muscles [76–82]. Of note, these abnormal peri- or intra-organ fat (APIFat) depositions have been linked to CV risk [83–89]. Therefore, this excessive “orthotopic” accumulation of adiposity in different organs/tissues in T2DM patients may be implicated in the development of diabetic vascular complications [90].
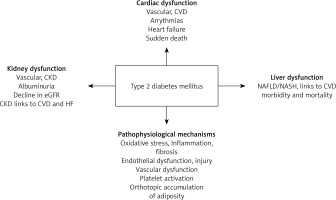
In conclusion, T2DM is characterized by cardiac, kidney and liver dysfunction, even upon its diagnosis (Figure 1). Based on these data, we propose that T2DM may represent a Cardiac-Kidney-Liver (CKL) syndrome rather than a simple metabolic disorder. Of note, very recently, the AHA suggested the existence of the cardiovascular-kidney-metabolic (CKM) syndrome that is defined as a health disorder characterized by interconnections between obesity, T2DM, CKD and CVD [91]. The recognition of T2DM as “CKL syndrome” can facilitate a better understanding of the underlying pathophysiological mechanisms of the disease development and complications, thus leading to more appropriate and effective treatments. This approach can enable the implementation of a ‘holistic person-centered’ interdisciplinary care for T2DM patients, according to the current guidelines [4]. In this context, certain antidiabetic drugs, namely sodium-glucose transport protein 2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists and dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 co-agonists represent ideal therapeutic agents that can target the whole spectrum of the CKL syndrome based on their beneficial cardiorenal effects and their benefits on MASLD [92–99].