Introduction
Recent epidemiological reports demonstrate that the incidence of hepatitis E virus (HEV) infections in Europe is constantly increasing. According to the recent Europe-wide study by Aspinall et al. the number of reported HEV cases in Europe increased from 515 in 2005 to 5167 in 2015 [1]. The most prevalent HEV genotype that occurs endemically in European populations is genotype 3 [2]. These infections occur mainly in patients without a history of travelling to tropical endemic countries. HEV-infections with genotype 3 and 4 are considered as zoonotic diseases, which might be transmitted through direct contact with infected animals or indirectly through ingestion of food products from domestic (swine) or wild (boars, deer) animals. In the recent meta-analysis by Hartl et al. among significant risk factors associated with anti-HEV-IgG positivity were older age, the type of assay used for detection of antibodies (Abs) and contact with animals/swine [3]. What is more important, there were over 40 reported cases so far of blood-transmitted HEV infections in Europe and Japan, which calls for increased concern [4, 5].
Hepatitis E virus infection is an important medical concern that may lead to acute hepatitis E with liver damage but also chronic hepatitis E that eventually might be followed by liver cirrhosis (LC) [6]. The latter complication affects mostly immunodeficient populations of solid-organ and bone-marrow recipients, patients with lymphoproliferative disorders, patients during chemotherapy or other immune suppressive treatments and patients with chronic liver diseases. Apart from life-threatening acute liver insufficiency, HEV infection may be associated with transplant rejection or lead to the progression of liver fibrosis [7, 8]. In patients requiring chemotherapy or other immune suppressive treatment, the development of HEV-associated acute hepatitis may defer or interfere with therapy for underlying disease. Furthermore, HEV infection is known to induce numerous extrahepatic manifestations including Guillain-Barré syndrome, neuralgic amyotrophy, glomerulonephritis, cryoglobulinemia, and pancreatitis [9].
The epidemiology of HEV infections may vary across the countries and specific regions in Europe and is largely dependent not only on actual prevalence but also on lack of routine testing, case definitions and reporting systems [1]. Countries with the highest prevalence of anti-HEV-IgG include France (especially the southwest) up to 52%, Germany approximately 29%, and the Netherlands 27% [3]. Interestingly, those countries are also important sources of agriculture. However, on the map of Europe displaying the distribution of HEV infections there are still regions of terra incognita, especially in eastern parts of the continent. Also in Poland there is still limited evidence on the prevalence of HEV infections. Alarming data come from the Institute of Hematology and Transfusion Medicine in Warsaw showing occurrence of anti-HEV-IgG in almost 43% of blood donors and, more importantly, HEV-RNA positivity in about 1 in 2200 donations. Analysis of the regional distribution revealed that anti-HEV-IgG seroprevalence varied across the country, with the lowest prevalence in north-eastern Poland in Bialystok (28.86%) and the highest in west-central Poland in Poznan (61.31%). Phylogenetic analyses of viremic cases showed HEV infection with zoonotic genotype 3 (subtype 3c and 3i) [10]. Furthermore, a cross-sectional study among 1021 hunters from west-central Poland showed anti-HEV-IgG prevalence of 22.1%. Importantly, data from risk groups of chronic HEV are missing. In a study from west-central Poland, by Bura et al., including 244 human immunodeficiency virus (HIV)-infected patients, anti-HEV-IgG was detected in 50.8% of patients by Wantai Assay, of whom more than half had never travelled abroad [11].
In the current study a large cohort of immunocompromised patients after solid-organ transplantations, HIV-infected patients, and patients with advanced liver diseases were screened for serological markers of HEV infection to reveal the burden of hepatitis E in north-eastern Poland.
Material and methods
Studied population
A cohort of 450 patients (mean age: 50.35 years, range: 21–80) from north-eastern Poland who presented at the university hospital center in the years 2013–2016 were enrolled in this study. Among them 180 persons were renal (n = 176) or liver (n = 4) transplant recipients (TR), 90 patients were HIV infected and 180 persons had confirmed LC of different etiology, mostly alcoholic liver disease (ALD) n = 117, hepatitis C virus (HCV) n = 18, mixed ALD/HCV n = 7, hepatitis B virus (HBV) n = 4, autoimmune hepatitis (AIH) n = 5, primary biliary cholangitis (PBC) n = 4, non-alcoholic steatohepatitis (NASH) n = 4, suspected congestive hepatopathy or unknown etiology n = 21. The samples were collected randomly in each selected patient group. The characteristics of the studied group are presented in Table I. Living in an urban area of residence was reported by 68% (65–70%) of patients across all studied groups. In the group of renal/liver TR the immunosuppressive regimens included tacrolimus (n = 115), cyclosporine (n = 58), everolimus (n = 5) and sirolimus (n = 2). All renal TR (176) underwent hemodialysis prior to kidney transplantation. In transplant patients no alanine transaminase (ALT) elevations were noted. The median (IQR) Child-Pugh score was 8 (7–10) and the Model of End-Stage Liver Disease (MELD) score was 16 (10–21) in patients with ALD. In LC of other etiologies the median Child-Pugh score was 8 (6–9) and the MELD score was 10 (7–12). In the cirrhotic group, 25 (13.9%) patients had confirmed HCV infection, while 4 (2.2%) were HBV-infected. Among HIV-positive patients 39 (43.3%) had HIV/HCV coinfection, whereas 2 had HIV/HBV coinfection (2.2%). The median (IQR) HIV viral load was 380 (19–44200) copies per ml and the median (IQR) CD4 count was 414 (225–567) cells/ml.
Table I
Characteristics of studied population (n = 450)
| Parameter | Transplant recipients | Liver cirrhosis | HIV (+)‡ (n = 90) | |
|---|---|---|---|---|
| Renal (n = 176) Liver (n = 4) | ALD† (n = 117) | Other (n = 63) | ||
| Age, mean ± SD [years] | 51.6 ±12.9 | 50.4 ±12.4 | 57.6 ±11.12 | 38.2 ±9.8 |
| Male sex, n; % | 96; 53 | 90; 77 | 38; 60 | 62; 69 |
| Alanine transaminase, median; IQR [U/l] | 25; 12–55 | 40; 23–65 | 53; 31–119 | 32; 20–58 |
Screening testing for serological markers of HIV, HBV and HCV infections and HIV RNA was conducted with Architect System (Abbott Laboratories, Illinois, USA). The study was carried out after its acceptance by the institutional bioethical committee of Medical University of Bialystok. All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was supported by an institutional scientific grant.
Determination of anti-HEV-IgG, IgM antibodies and HEV antigen in serum samples
The detection of serum anti-HEV-IgG, IgM Abs and HEV-antigen (HEV-Ag) was performed by the ELISA method with commercially available diagnostic kits containing HEV recombinant antigens binding to the serum IgG Abs (Wantai HEV-IgG ELISA, Wantai, China), IgM Abs (Wantai HEV-IgM ELISA, Wantai, China) and pre-coated Abs binding to serum HEV-Ag (Wantai HEV-Ag ELISA Plus, Wantai, China). The sensitivity of HEV-IgG, HEV-IgM and HEV-Ag ELISA kits according to the manufacturer was 93.33–97.96%, 97.1% and 66.7%, respectively. The specificity of ELISA kits used in this study was estimated by the manufacturer as 99.99% for HEV-IgG, 98.4% for HEV-IgM, 99.93% for HEV-Ag.
Statistical analysis
Data are presented as median (IQR) unless stated otherwise. For comparisons of the groups, χ2, Fisher exact and Mann-Whitney U tests were performed when appropriate. Values of p < 0.05 were considered statistically significant. Statistica 12 for Windows was used to perform the analysis (StatSoft Inc., Tulsa, USA).
Results
Prevalence of anti-HEV-IgG and IgM antibodies and HEV Ag in studied cohort
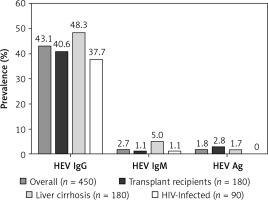
The overall prevalence of anti-HEV-IgG Abs in the studied population was 43.1% (194 of 450). The frequency of detection of anti-HEV-IgM Abs was 2.7% (12 of 450). HEV-Ag was detected in 8 (1.8%) cases. In group-based analyses, the highest prevalence of IgG HEV was found in TR and patient with LC, the lowest in the HIV-infected population; however, the difference was non-significant (NS) (Figure 1).
The analysis of risk factors for HEV infection revealed that anti-HEV-IgG positivity was associated with older age (p = 0.0004) (Table II). Moreover, male gender was associated with significantly increased risk for HEV infection as the male patients were found to be more frequently anti-HEV-IgG positive compared to females – 45.1% vs. 33.5% (p = 0.02) (Table II). The anti-HEV-IgG seroprevalence in patients with increased ALT activity vs. the group with normal ALT activity was similar, 43.63% vs. 51.28% (NS). None of the patients presented symptoms of acute hepatitis at the time of study enrolment.
Table II
Associations between selected factors and prevalence of anti-HEV-IgG in studied populations (n = 450)
| Factor | Number tested | Anti-HEV-IgG† positive, n (%) | OR | 95 CI | P-value |
|---|---|---|---|---|---|
| All study participants | 450 | 194 (43.11) | – | – | – |
| Risk group: | |||||
| HIV-infected‡ | 90 | 34 (37.77) | 1 | ||
| Transplant recipients | 180 | 73 (40.55) | 1.124 | 0.67–1.89 | 0.694 |
| All liver cirrhotics | 180 | 87 (48.33) | 1.541 | 0.92–2.58 | 0.119 |
| Alcoholic liver cirrhotics | 117 | 61 (52.13) | 1.794 | 1.02–3.14 | 0.049 |
| Male gender | 286 | 129 (45.10) | 1.576 | 1.052–2.36 | 0.02 |
| Urban place of residence | 118/180 | 57 (48.31) | 0.934 | 0.49–1.79 | 0.869 |
| Age group: | 0.0004 | ||||
| 21–30 | 35 | 7 (20.00) | 1 | – | – |
| 31–40 | 92 | 34 (36.95) | 2.345 | 0.92–5.94 | 0.089 |
| 41–50 | 83 | 31 (37.35) | 2.385 | 0.93–6.11 | 0.085 |
| 51–60 | 125 | 63 (50.40) | 4.065 | 1.65–9.99 | 0.002 |
| > 60 | 86 | 50 (58.14) | 5.556 | 2.19–14.12 | 0.0001 |
| Renal/liver transplant recipients – type of immunosuppressive regimen | 180 | ||||
| Cyclosporin | 58 | 19 (30.76) | 0.927 | 0.49–1.74 | 0.874 |
| Tacrolimus | 115 | 40 (34.78) | 1.015 | 0.62–1.66 | 0.953 |
| Everolimus | 5 | 2 (40.00) | 1.269 | 0.21–7.79 | 0.797 |
| Sirolimus | 2 | 1 (50.00) | 1.903 | 0.12–30.97 | 0.646 |
| Liver cirrhosis: | 180 | ||||
| Etiology – alcoholic (vs. other etiologies of liver cirrhosis) | 117 | 61 (52.14) | 1.55 | 0.83–2.88 | 0.211 |
| Model of End Stage Liver Disease [points]: | 0.378 | ||||
| < 9 | 39 | 18 (46.15) | 1 | – | – |
| 10–19 | 74 | 37 (50.00) | 1.167 | 0.54–2.54 | 0.843 |
| > 20 | 34 | 21 (61.76) | 1.885 | 0.74–4.80 | 0.241 |
| Child-Pugh classes: | 0.611 | ||||
| A | 32 | 17 (53.12) | 1 | – | – |
| B | 68 | 38 (55.88) | 1.118 | 0.48–2.59 | 0.832 |
| C | 31 | 14 (45.16) | 0.727 | 0.27–1.96 | 0.617 |
| HIV-infected‡: | |||||
| CD4: | 0.265 | ||||
| < 200 | 17 | 5 (29.41) | 1 | – | – |
| 200–500 | 36 | 18 (50.00) | 2.400 | 0.70–8.22 | 0.236 |
| > 500 | 29 | 10 (34.48) | 1.263 | 0.35–4.61 | 0.723 |
| HIV RNA [copies/ml]‡: | 0.591 | ||||
| < 20 | 29 | 12 (41.38) | 1 | – | – |
| 20–1000 | 16 | 8 (50.00) | 1.42 | 0.42–4.84 | 0.755 |
| > 1000 | 37 | 13 (35.14) | 0.77 | 0.28–2.09 | 0.620 |
| HIV/HCV§ vs. HIV‡ monoinfection | 39 | 15 (38.46) | 0.903 | 0.37–2.18 | 0.820 |
HEV seroprevalence in solid organ transplant recipients
The prevalence of anti-HEV-IgG, IgM Abs and HEV-Ag is presented in Figure 1. Two patients after renal transplantation had detectable HEV Ag and anti-HEV-IgG in serum. Both of them had normal ALT activity. None of the liver TR had detectable anti-HEV-IgG, anti-HEV-IgM or HEV-Ag in serum. Prevalence of HEV-Ag, anti-HEV-IgG and IgM in TR was not associated with the type of the immunosuppressive regimen (Table II).
HEV seroprevalence in patients with liver cirrhosis
The analysis of the presence of serological markers of HEV infection with regard to the etiology of LC revealed higher anti-HEV-IgG prevalence in patients with ALD (52.1%, n = 61/117) compared to other etiologies (41.3%, n = 26/63), but the difference was non-significant (NS). The frequency of anti-HEV-IgM was 6.8% (n = 8/117) in ALD vs. 1.6% (n = 1/63) in LC of other etiologies (NS). HEV-Ag was detected in 1.7% (n = 2/117) vs. 1.6% (n = 1/63) (NS), respectively. In the second large cirrhotic group of 25 HCV-infected patients (HCV-18, HCV/ALD-7) the prevalence of anti-HEV-IgG Abs was 40% (n = 10/25). None of the patients with laboratory markers of acute liver injury had anti-HEV-IgM or HEV-Ag detectable in serum samples. The risk for anti-HEV-IgG seropositivity was almost double in patients with MELD > 20 points in comparison to the group with MELD < 9 points (OR 1 vs. 1.88, NS); however, the difference was not statistically significant (Table II).
HEV seroprevalence in HIV-infected population
Overall prevalence of anti-HEV-IgG in HIV-infected patients was 37.7%. There was no statistically significant difference in the prevalence of anti-HEV-IgG depending on the number of CD4 cells or HIV viral load. Furthermore, there was no statistically significant risk associated with HIV/HCV coinfection vs. HIV-monoinfection (Table II).
Discussion
The frequency of HEV infections expressed as anti-HEV-IgG seroprevalence in European countries varies across the region and is estimated from 0.6 to over 52%. Anti-HEV-IgG Abs appear/reappear in serum after primary or secondary HEV infection. The data on the time of persistence of anti-HEV IgG Abs after HEV infection is scarce and inconsistent. However, recent evidence shows that in some patients after several years seroreversion may occur [12]. Nevertheless, testing for anti-HEV Abs is the most important screening evaluation for HEV infection. Diagnostic tests used currently for assessment of the presence of anti-HEV Abs have different sensitivity [3]. For the purpose of this study, we chose Wantai (China) diagnostic assays, which seem to provide a high sensitivity and specificity profile in independent studies. The specificity of HEV IgG ELISA was estimated as 97.8–100%, whereas sensitivity varied from 45% in acute hepatitis E in immunocompromised individuals, 76.2% in solid organ TR to 93.2–100% in the immunocompetent group. As reported by Rossi-Tamisier et al., the concordance rate of Wantai anti-HEV IgG with immunoblot testing was estimated as 80%. The sensitivity of 74–97.1% and specificity of 99–100% were evaluated for HEV-IgM ELISA [13–17]. Nevertheless, the specificity of 99.93% along with the sensitivity of 66.7% for HEV-Ag (vs. HEV-RNA detection by PCR) might have led to underdetection of actual viremic HEV cases. On the other hand, in the study of Mishra et al. in patients with HEV-related acute viral hepatitis and acute liver failure the concordance between HEV-Ag ELISA and HEV RNA was about 75%. Therefore, HEV-Ag was proposed as a cheap, valuable marker of active viremic HEV infection especially in patients in the serological window, in HEV reinfection, in pregnant and immunocompromised patients who do not produce IgM Abs [18].
Recent work of Grabarczyk et al. indicates high endemicity of HEV infections in blood donors in Poland. The numbers indicate that 43.52% of the healthy population have been infected with HEV in the past, as HEV RNA positivity is scarce. In the region of north-eastern Poland, where our study was conducted, the reported anti-HEV IgG seroprevalence was 28.86% and was lowest in Poland [10]. In our study we found anti-HEV IgG seropositivity in 43.11% of enrolled patients, which seems to be considerably higher in comparison to the blood donor population.
The population of solid-organ TR is at most risk of development of chronic HEV infection. According to the data collected by Kamar et al., chronic HEV infection might affect as many as 60% of solid organ TR. Those who receive tacrolimus-based immune suppression were more prone to develop chronic HEV infection. Interestingly, decreasing the dosage of tacrolimus reduced the frequency of chronic HEV development by 30% [19]. The anti-HEV-IgG seroprevalence in our population of solid organ TR was 34%. It was considerably higher than the anti-HEV-IgG seropositivity rates, ranging from 4.4% to 20.9%, reported in solid organ TR by other authors [20–24]. There were no differences across the groups with reference to specific immune suppressive regimen. The important limitation of our study was the lack of HEV RNA assessment in HEV IgG seropositive patients. However, the available evidence suggests 75% agreement between HEV-Ag and HEV RNA testing [18]. We reported HEV-Ag presence in 2.8% of TR. In several studies in solid organ TR, active HEV replication reflected by the HEV RNA positivity in serum samples varied from 1.3% to 2.1% [20, 21, 25, 26]. These results identify the solid organ TR as a vulnerable group for the development of chronic HEV infection. Taking together the high seroprevalence of anti-HEV-IgG and rather low anti-HEV-IgM and HEV-Ag, it seems that in Poland HEV infections might be quite common but underdiagnosed.
There are limited data on the occurrence of HEV infections in patients with chronic liver diseases. Pischke et al. found HEV RNA positivity in only one patient in a group of 208 subjects with AIH and no associations between HBV or HCV concurrent infections [27]. Alcohol abuse and associated behavior might be a considerable risk factor for acquiring HEV infection, as the anti-HEV-IgG seropositivity rate in the study of Vilibic-Cavlek et al. in selected population groups in Croatia was the highest in alcohol abusers (8.9%), exceeding rates reported from the general Croatian population (5.6%) [28]. In our study in patients with ALD we found high prevalence of anti-HEV-IgG Abs reaching 52.13%, almost 2-fold higher than that found in blood donors in north-eastern Poland as reported by Grabarczyk et al. [10]. The prevalence of HEV-Ag in our patients with ALD was 1.7%, which may suggest persistence of HEV infection in this group.
The seroprevalence of anti-HEV-IgG in HIV-infected patients was estimated from 5% to 7.3% in several studies from southern and western Europe, and was comparable to rates reported for the general population. Interestingly, none of the HIV-infected patients has detectable HEV RNA [29–31]. In contrast, data from Russia and Belarus revealed significantly increased anti-HEV-IgG seroprevalence in HIV-infected patients (11%), over 5-fold higher than values reported for the general population, and particularly high in patients with developed acquired immunodeficiency syndrome (40%) [32]. The seroprevalence of anti-HEV-IgG in HIV-positive patients in west-central Poland was estimated as 50.8% and was comparable to the prevalence reported in blood donors – 49.6% [11]. In our study, in the HIV-infected population from north-eastern Poland the seroprevalence of anti-HEV-IgG was 37.7% and slightly exceeded the figure reported by the Regional Blood Transfusion Center (28.86%) [10]. None of the patients was positive for HEV-Ag (Figure 1). There was no association between HEV-IgG seropositivity and CD4 count or HIV viral load. These observations are in line with other authors and suggest that HIV infection seems to have no significant impact on the development of chronic HEV infection.
In line with available studies in Polish populations, by Bura et al. and Grabarczyk et al., we also found significant positive correlations between the anti-HEV-IgG seroprevalence and age [10, 11]. This probably results from the increasing risk of environmental/occupational exposure to HEV with the increase of age. The significantly predominant anti-HEV IgG seropositivity in male gender vs. female is difficult to explain, but it is in concordance with the report of Grabarczyk et al. and previously published data from other authors [10, 33].
Our study is among the first initial reports on the seroprevalence of HEV markers in the Polish population. However, it is important to remark that the lack of HEV RNA assessment appears to be their major limitation. Considering the quite high seroprevalence of anti-HEV-IgG in the studied groups, further broad screening of the population is needed with the support of molecular methods for regular bedside diagnostic purposes.
In conclusion, in this largest HEV epidemiological study from Poland including vulnerable groups of patients, the prevalence of anti-HEV-IgG among solid organ TR, cirrhotic patients and HIV-infected subjects is significantly higher in comparison to the data reported from other Central and Western European countries. Taking into account the high seroprevalence of anti-HEV-IgG and rather low anti-HEV-IgM, it seems that in Poland HEV infections might be quite common but underdiagnosed and further screening actions and rising of awareness are mandatory.