The large number of diagnoses of lung diseases results in the appearance of different aspects of cures for pulmonary diseases. Decellularized lung tissue can be used in the laboratory to study various aspects of pulmonary biology and physiology [1]. Recently, a new strategy has been proposed for the cellular engineering of lung tissue [2]. The main components are three-dimensional (3D) scaffolds [3], a system that supports active 3D composite biology [4] and a source of stem cells [5]. Biochemical and basic, peripheral examinations are still performed and are needed. For example, peripheral diagnostic biomarkers are useful for detecting hypertension [6]. Chronic obstructive pulmonary disease (COPD) is a widely diagnosed lung disease worldwide [7]. The important inflammation-related part of the COPD mechanism is mostly understood. COPD is characterized by increased levels and activity of different immune cells that secrete a variety of inflammatory mediators, including cytokines. A prominent role is played by interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, which are known as master cytokines [8]. The presence of COPD reduces the quality of life. In particular, measuring quality of life is suggested for older individuals [9]. The presence of disturbances in mental health, including depressive symptoms, and inflammation play important roles in lowering the quality of life for COPD patients [10]. Studies involving patients diagnosed with depression have found significant elevations of pro-inflammatory cytokines in plasma and/or serum levels that resulted in the formulation of the “cytokine hypothesis of depression” [11]. The main cytokines related to depression include IL-1, IL-6, TNF-α, and IFN-γ [11]. The occurrence of depressive symptoms may result from direct inflammatory-related pathways, but this is not the only possibility.
Both COPD and depressive disorder are characterized by disturbances in thyroid hormone levels [12, 13] affected by iodothyronine deiodinases (DIOs), among other factors.
Recently, the deiodinase iodothyronines (DIOs) have gained a “reawakened” interest as an immune-inflammatory marker that can be induced and/or interfere with cytokines. A correlation between cytokines and deiodinase expression levels has been found.
For example, IL-1, IL-6, and TNF-α affect T3-mediated induction of mRNA for deiodinase type 1 (DIO1) [14]. Recently, the presence and subcellular location of DIO3 at the transcriptional level have been confirmed in human neutrophils [15]. DIO2 is known to be up-regulated in inflammation [16], but its activity also is needed to determine proper levels of triiodothyronine in the brain, while low activity of DIO2 and T3 level are associated with the presence of depressive symptoms [17].
Depressive disorder, similar to COPD, is one of the most common diseases and imposes a heavy burden on individuals and society [18]. Considering the frequent presence of depressive symptoms in COPD patients, there is an unmet need to explain the mechanisms of action and identify the molecules that link COPD and depression. Thus, peripheral biomarkers could be used to determine the risk of depressive symptoms in COPD patients.
The aim of this study was to evaluate the levels of inflammatory and thyroid hormone metabolism-related molecules that may explain the possibility of the presence of depressive symptoms in COPD patients. We measured and compared the profiles of four main cytokines, (master cytokines) and DIOs, between COPD and recurrent depressive disorder (rDD) patients and healthy controls
Methods
Subjects
A group of 23 patients (9 women, 14 men) with stable COPD (mean (SD), median (IQR) age 67.3 (10.02); 67 (63–71)), 27 patients (9 women and 19 men) with rDD (mean (SD), median (IQR) age, 57.4 (3.9), 57 (55–60)) and 25 (9 women, 17 men) healthy controls (age: mean (SD) 52.3 (6.7), median (IQR) 57 (43–60)) participated in the study. Both COPD and rDD were diagnosed according to the ICD-10 criteria. For a detailed description of patients and controls see Appendix 1. For cytokine and deiodinase iodothyronine measurements see Appendix 2. For statistical analysis see Appendix 3.
Results
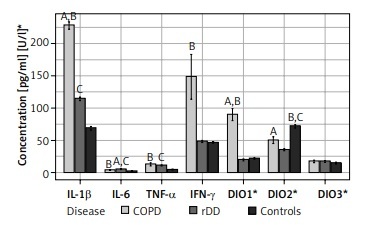
IL-1β was highest in COPD patients (229.9, IQR 214.0–248.9) and differed significantly when this group was compared with rDD patients (116.4, IQR 106.3–124.7) and controls (64.24, IQR 58.9–81.3), p < 0.001. There was a significant difference in the concentration of IL-1β between rDD patients and controls, p < 0.001 (Figure 1).
Figure 1
Distribution of concentrations of the seven parameters studied by type of disease and indication of significance of differences between groups (“A” above the bars indicates significant difference between COPD and rDD; “B” indicates significant difference between COPD and Controls; “C” indicates significant difference between rDD and Controls. A bold italic capital letter means a significance level of p < 0.001, an italic capital letter without bold means a significance level of p < 0.010, a capital letter without italics and without bold means a significance level of p < 0.050. Letters were given for the group with the higher concentration)
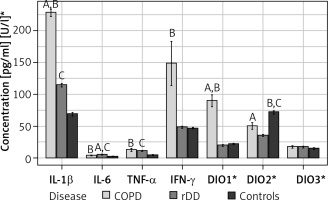
IL-6 concentrations were significantly higher in COPD (3.9, IQR 3.1–4.7) and rDD (5.0, IQR 4.7–6.2) patients than in controls (1.72, IQR 1.4–2.6), p < 0.001. IL-6 levels were highest in the rDD group and differed significantly when compared to the COPD group, p = 0.007 (Figure 1).
Concentrations of TNF-α were significantly higher in COPD patients (8.5, IQR 5.5–15.1) and in rDD patients (11.25, IQR 10.3–12.3) compared to controls (4.6, IQR 4.1–5.4), p < 0.001. There was no significant difference between COPD and rDD, p > 0.05 (Figure 1).
Concentration of IFN-γ was not significantly higher in COPD patients (81.3, IQR 42.4–210.2) when compared to rDD patients (47.6, IQR 42.7–53.6), p = 0.1, but was significantly different when compared to controls (45.3, IQR 42.9–49.1), p = 0.025. IFN-γ concentration did not differ significantly between rDD patients and controls, p > 0.05 (Figure 1).
The highest presence of DIO1 was in COPD patients (95.6, IQR 48.2–115.8) and differed significantly between rDD patients (17.34, IQR 15–24.1) and controls (21.34, IQR 17.9–25.1), p < 0.001. There was no difference between rDD and controls, p > 0.05 (Figure 1).
The DIO2 value was higher in COPD patients (53.1, IQR 30.4–64.3) and differed significantly from rDD patients (36.0, IQR 31.9–38.2), p = 0.02. The highest value was in the controls (67.6, IQR 63–80.2) and differed significantly from COPD patients, p = 0.003, and rDD patients, p < 0.001 (Figure 1).
There were no significant differences in DIO3 values between the three investigated groups: COPD patients (18.4, IQR 9.9–27.7), rDD patients (15.44, IQR 11.5–24.5), and controls (12.9, IQR 9.1–19.9), p > 0.05 (Figure 1).
In rDD patients, assessment of the relationship revealed a correlation between DIO2 and IFN-γ (R = 0.47, p = 0.01) and a correlation between DIO3 and IL-6 (R = 0.4, p = 0.04).
We did not observe any other correlation.
Discussion
To the best of our knowledge, there is only one published study comparing the inflammatory statuses between COPD patients and those with depressive disorder [19]. Our study provides novel information and confirms potential inflammation-related and thyroid hormone-related mechanisms involved in the development of depressive symptoms in COPD patients. In addition, the results of our study identify molecules that might participate in the development of depressive symptoms in COPD patients.
IL-1β is a product of pro-IL-1β activation by caspase-1. The expression and activity levels of caspase-1 are promoted after processing by the nucleotide oligomerization binding domain-like receptor protein (NLRP3) inflammasome complex, which generates biologically active IL-1β [20]. Thus, the expression and activity levels of caspase-1 may determine IL-1β production and differentiate between COPD and rDD patients. In this case, the inflammasome is activated when pathogen-associated molecular patterns (PAMPs) are involved. PAMPs are important inducers of inflammatory pathways in COPD [20], but few have been investigated in depressive disorder. Increased levels of IL-1β, in particular, have been associated with increased inflammasome activation in COPD patients [21].
The increased levels of IL-1β in COPD have been associated with increased inflammasome activation in COPD patients [21], while the mRNA levels of AIM 2, a component of inflammasomes that can activate caspase-1, were similar between individuals with depressive symptoms and non-depressed participants [22]. The IL-1β precursor is processed by other serine proteases, including neutrophil proteases, such as elastase, chymase, and granzyme A. Regarding IL-1β formation, one explanation for the differences between COPD and rDD is that they may result from neutrophil infiltration and/or the levels and activity of the abovementioned proteases [23].
The differences in the levels of IL-1β between COPD and rDD patients may result from high airway cell secretion and increased expression levels of systemic IL-1β. Many other factors influence IL-1β levels. For example, IL-1β expression can be promoted by the resistin-like molecule, which is highly expressed in COPD patients [24]. Tissue and cell distribution may also play a role in the determination of its expression level. Differences between lung and brain distribution may influence the systemic levels, as well as the gut-lung axis and gut-brain axis. The microbiome, the gut-lung axis and the gut-brain axis may influence inflammation, as the microbiome can affect the NLRP3 inflammasome response and IL-1β expression [25].
IL-6 has pro- and anti-inflammatory activities. Higher IL-6 levels in rDD may reflect the anti-inflammatory pathway of Il-6 signaling. Patients in our study were treated with antidepressants with anti-inflammatory properties [26]. Kubera et al. [27] found that antidepressants increase IL-6 in animal models and in humans with depression.
Regarding the above, the differences in the levels of IL-6 between COPD and rDD patients may result from multidirectional processes. High IL-6 levels in rDD patients may reflect the anti-inflammatory pathway of Il-6 signaling. This suggestion can be supported by the fact that the patients included in our study were treated with antidepressants that have anti-inflammatory properties [26]. Antidepressants have been found to significantly decrease serum soluble IL-6R levels and IL-6 trans-signaling pathways [28]. In our study, higher levels of IL-6 in depression patients than in COPD patients may indicate a potential protective role of IL-6 in brain cells. This may influence peripheral levels due to bidirectional communication between the central nervous system and the periphery.
TNF-α is an important cytokine as its actions are numerous and diverse. TNF-α is mainly produced by macrophages. Many inflammatory mediators stimulate the secretion of TNF-α, for example lipopolysaccharide (LPS), IL-1β, IFN-γ as well TNF-α itself [29].
The differences in the levels of TNF-α between COPD and rDD were not significant.
Such results may suggest that the TNF-α-related pathway is similarly involved in both diseases and that TNF-α can be a risk factor for the possible development of depressive symptoms in COPD patients. Activation of intracellular signals through the TNF receptor is important in the production of pro-inflammatory cytokines, chemokines, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and TNF-α itself [29]. Considering the above, studies including anti-TNF-α therapy might be conducted, regarding the possibility of depression treatment in COPD patients.
We observed significantly higher IFN-γ levels in COPD patients compared to rDD patients and the controls and did not confirm the results of Rybka et al. [19], who suggested IFN-γ as a link between COPD and rDD. IFN-γ levels are highly increased in COPD patients during exacerbation but have also been observed to be lower in stable COPD patients than in control individuals [30]. IFN-γ levels were associated with Haemophilus influenzae colonization in COPD patients [31]. IFN-γ also plays an important role in depression. It has been observed that the administration of IFN-γ in humans produces symptoms of depression, and lymphocytes from depressed patients release more IFN-γ than those from healthy control individuals [32]. The explanation for the high IFN-γ levels in rDD patients presented by Rybka et al. [19] can be related, for example, to increased glucocorticoid signaling and activity of the HPA axis [33].
COPD patients had the highest levels of DIO1 when compared to rDD patients and the controls, which cannot be explained by the correlation with the investigated cytokine. The above results have not confirmed previous findings about the ability of cytokines to reduce the expression of DIOs. DIO2 levels were found to be significantly lower in COPD and rDD patients than in the controls and may suggest the molecule involved in the development of depressive symptoms in COPD, not only by inflammatory-related mechanisms but also by the influence of thyroid hormone levels or even other unexplored mechanisms. DIO2 is a determinant of the tissue-specific levels of T3, including those in brain areas [34].
The inducible nature of DIO2 during inflammation has also been reported [35]. The results of our study may not suggest a possible direct role of DIO2 as an inflammation-related molecule in COPD and rDD. Our data are opposite to the findings of Kwakkel et al., who observed increased expression of DIO2 during acute and chronic inflammation. Lower levels of DIO2 may result in lower T3 levels in the brain. Decreases in DIO2 in COPD and rDD patients may suggest the possibility of the presence of depressive symptoms in COPD patients as a result of lower levels of T3, especially in the brain. This should be considered a risk factor for the development of depressive symptoms in COPD patients.
In addition, in cases with microbial infection, lower thyroid hormone (TH) levels may not reduce bacterial inflammation, as TH are able to induce processes leading to bacterial killing [36].
DIO3 is the main determinant of rT3 but DIO3 involvement in inflammation is described.
For example, DIO3 is highly expressed in inflammatory lesions [37]. Nevertheless, our study did not confirm the role of DIO3 in inflammatory related diseases such as COPD and rDD.
In conclusion, IL-1β, TFN-α, and DIO2 might be suggested as putative agents that may be risk factors for the possible development of depressive symptoms in COPD patients. Patients with COPD having high levels of IL-1β and TNF-α and lower levels of DIO2 compared to healthy controls are suggested to be examined for the presence of mood disorders including depressive symptoms.
There are some limitation to this study. It is a single center study with a relatively small sample size, which might represent sampling bias. Obesity was not estimated in the participants and obesity was not an exclusion criterion. The smoking status was not evaluated in the whole group of participants. Patients included in the study were under medication, which might have affected the results. The results of this study showed significant differences but are of preliminary nature and should be interpreted with caution. The number of patients per group needs to be increased and more exclusion criteria should be used for further studies.